Figure 1. Current clinical protocols for adoptive cell therapy.
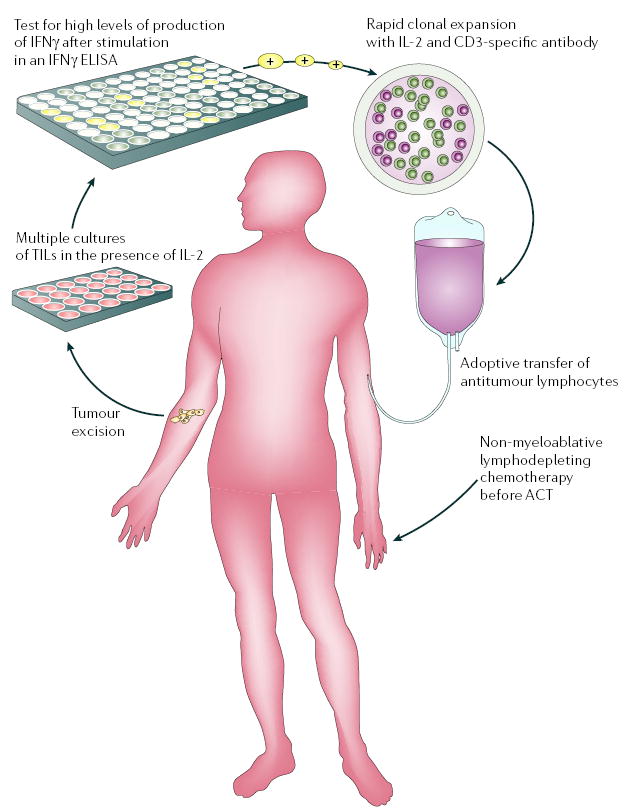
Adoptive cell therapy (ACT) requires the generation of highly avid tumour-antigen-reactive T cells. Tumour-specific T cells, derived from tumour-infiltrating lymphocytes (TILs), can be efficiently isolated ex vivo from melanoma lesions using high levels of interleukin-2 (IL-2). TILs are successively selected for their ability to secrete high levels of interferon-γ (IFNγ) when cultured with autologous or allogeneic MHC-matched tumour-cell lines. Alternatively, cell-mediated lysis has been used to identify tumour-reactive T cells for transfer. Highly avid, tumour-antigen-reactive T-cell populations selected for ACT are rapidly expanded (to up to 1011 cells) using CD3-specific antibody, exogenously supplied IL-2 and irradiated allogeneic peripheral-blood mononuclear ‘feeder’ cells, and are validated for activity before transfer. Patients now receive systemic immunosuppression before the adoptive transfer of antitumour lymphocytes. Published lymphodepleting regimens consist of a non-myeloablative, but lymphodepleting, conditioning chemotherapy comprised of cyclophosphamide and fludarabine before administration of T cells. Newer, as yet unpublished, regimens also include total body irradiation. ELISA, enzyme-linked immunosorbent assay. This figure is reproduced with permission from REF. 12 © (2005) Elsevier Science.
