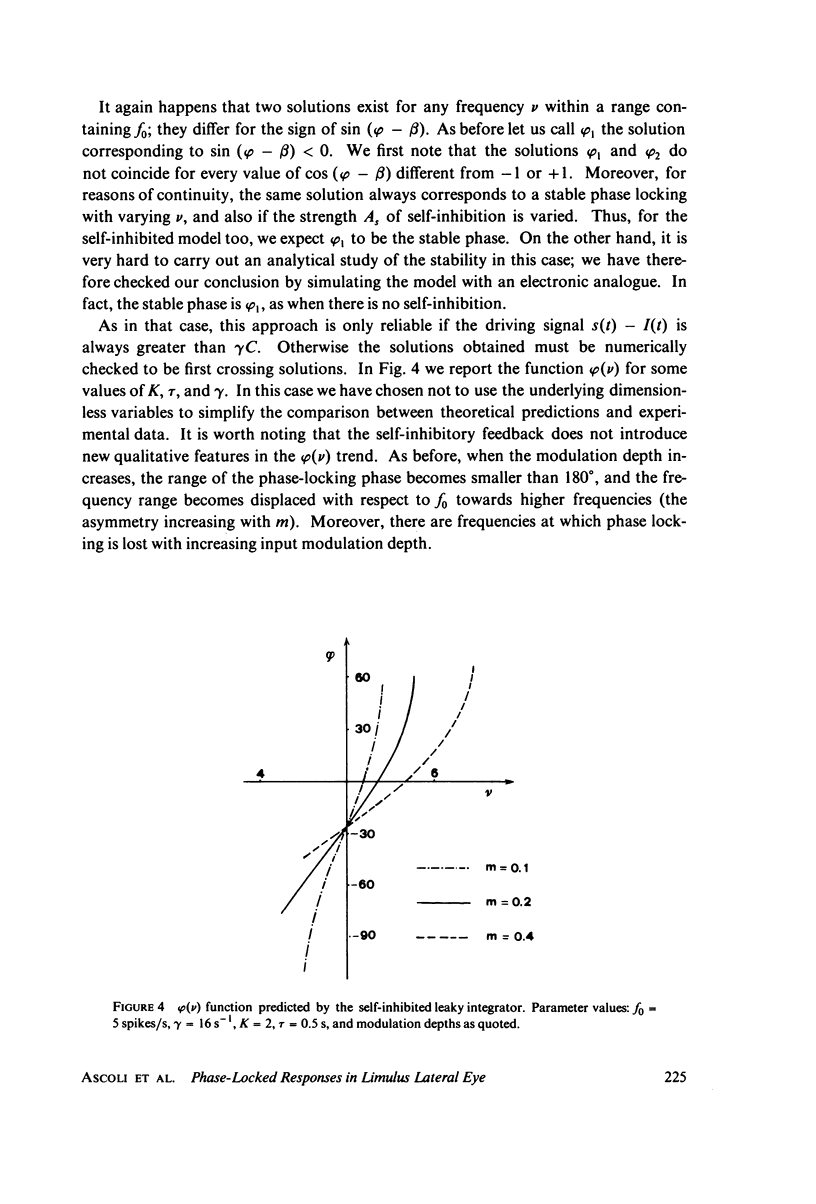
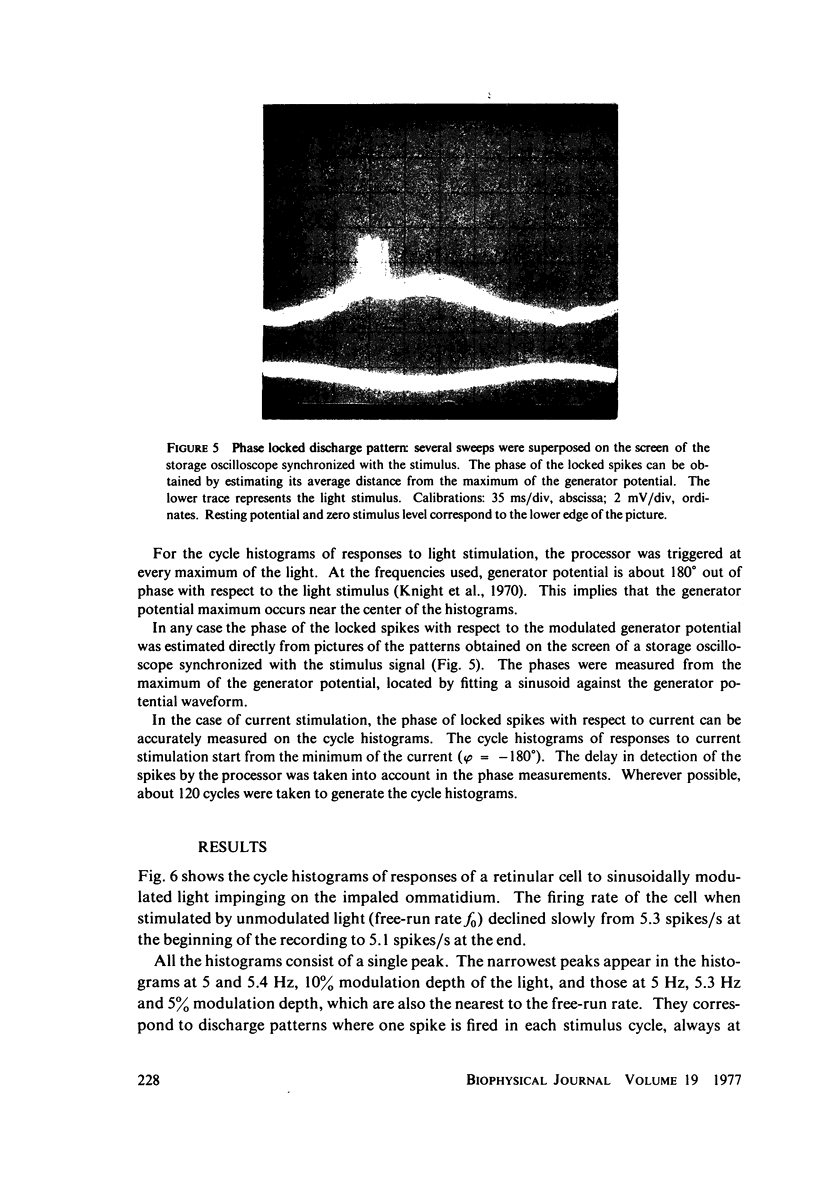
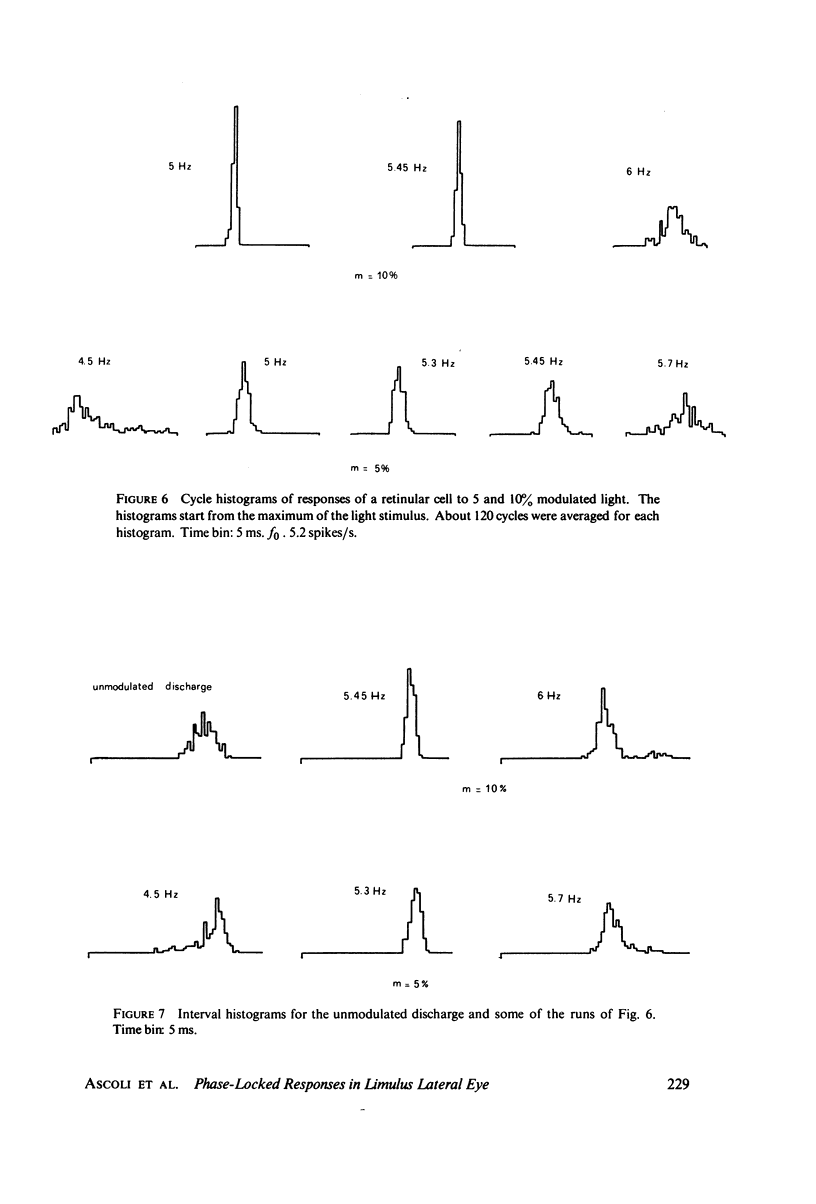
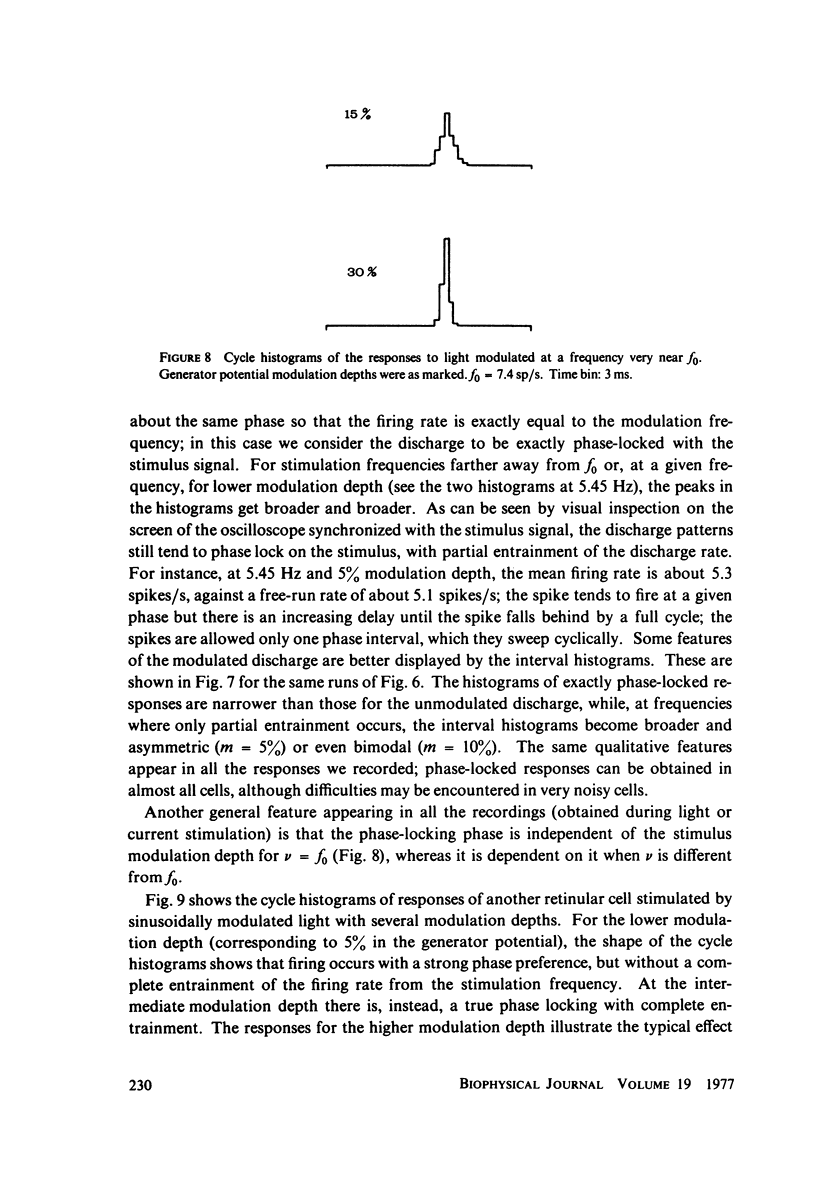
Abstract
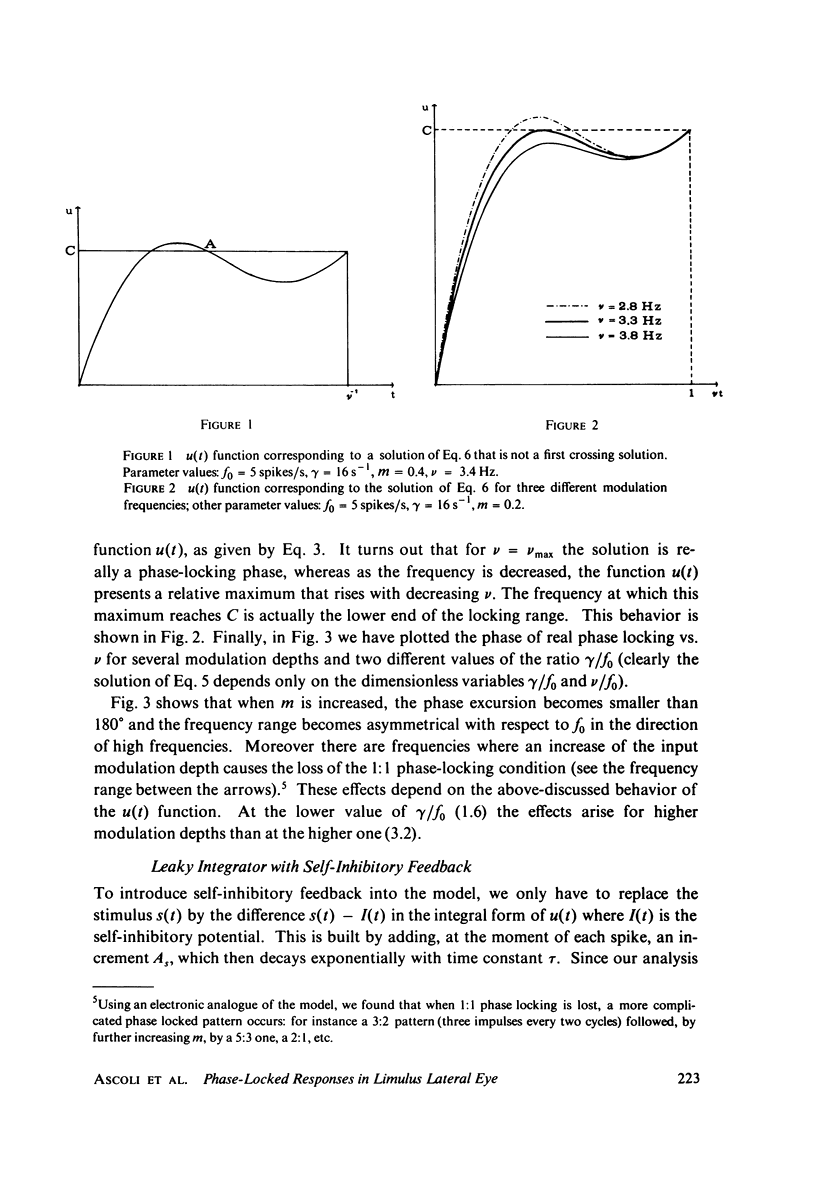
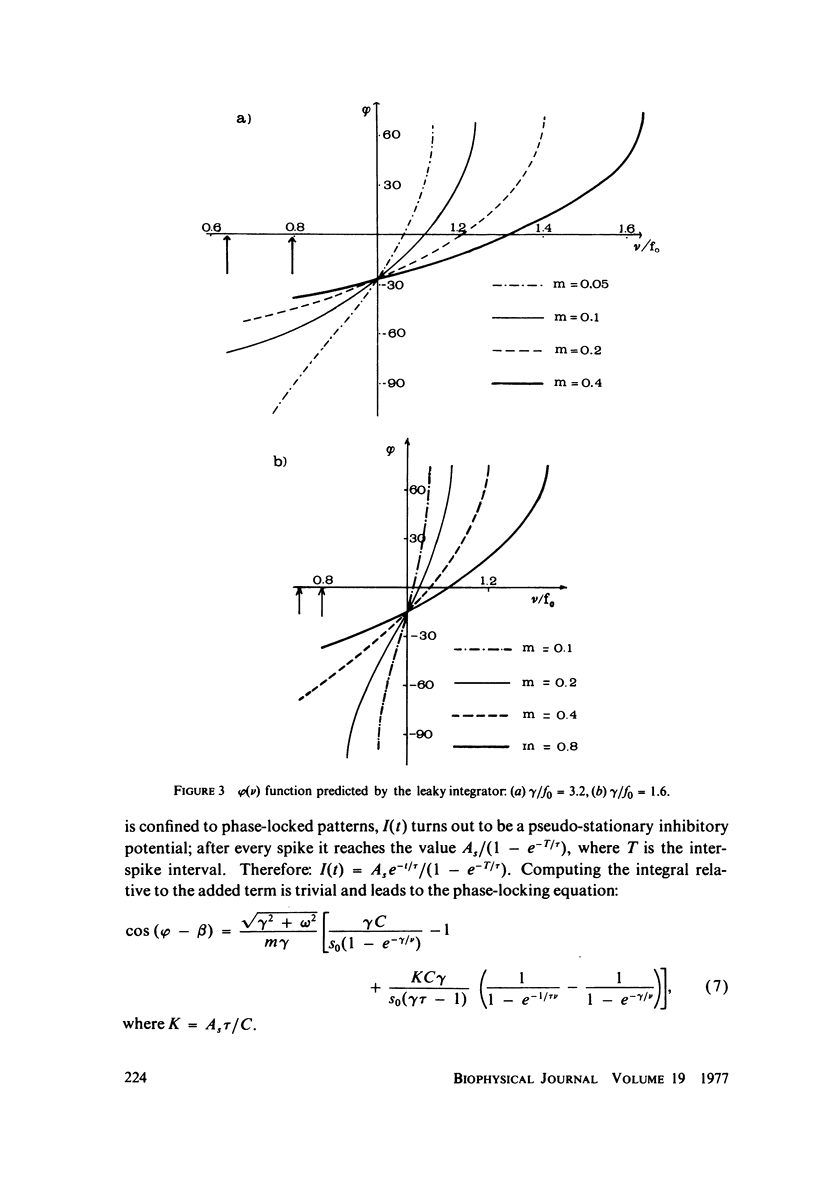
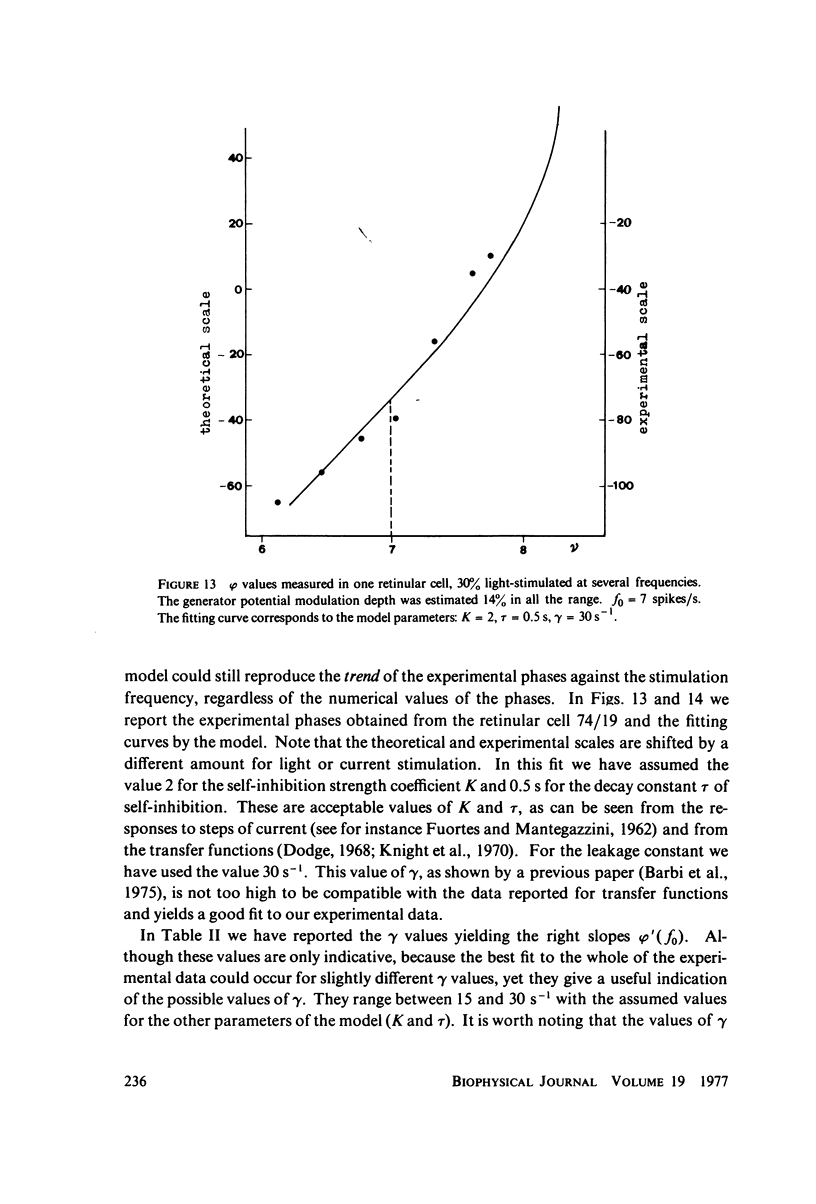
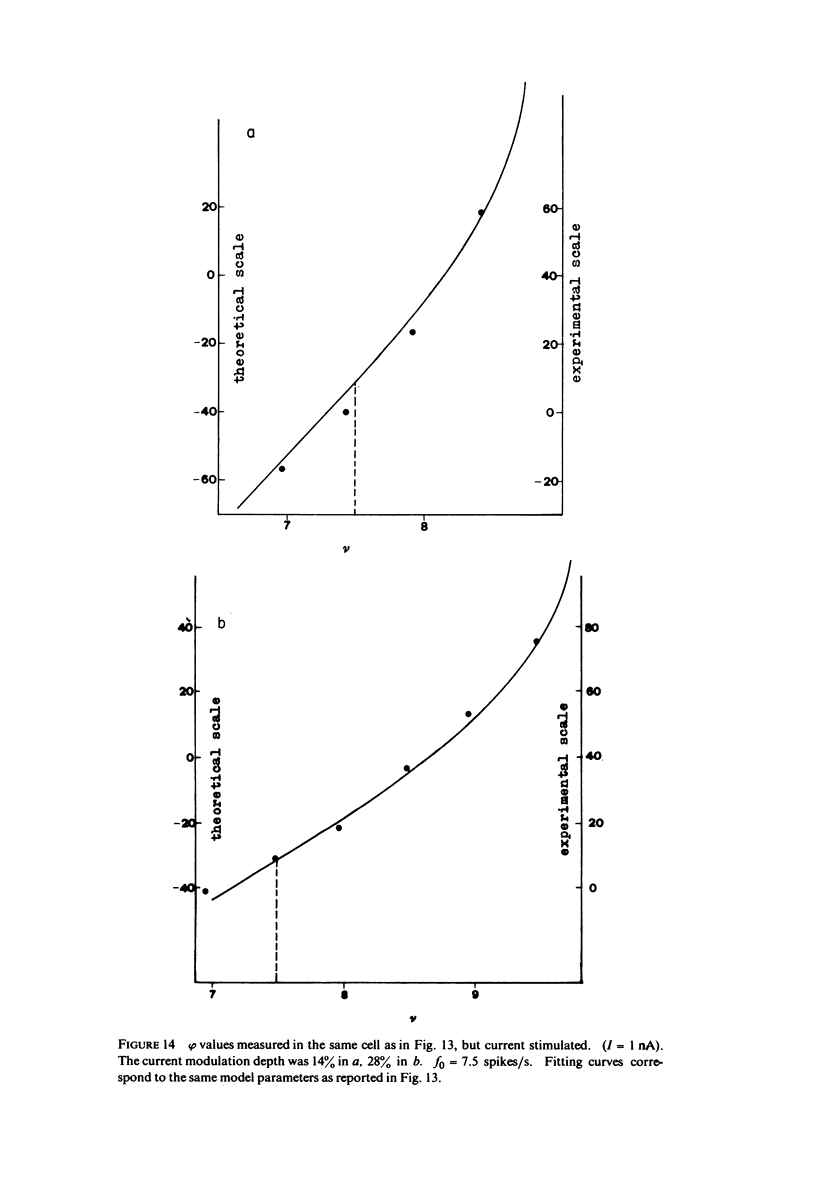
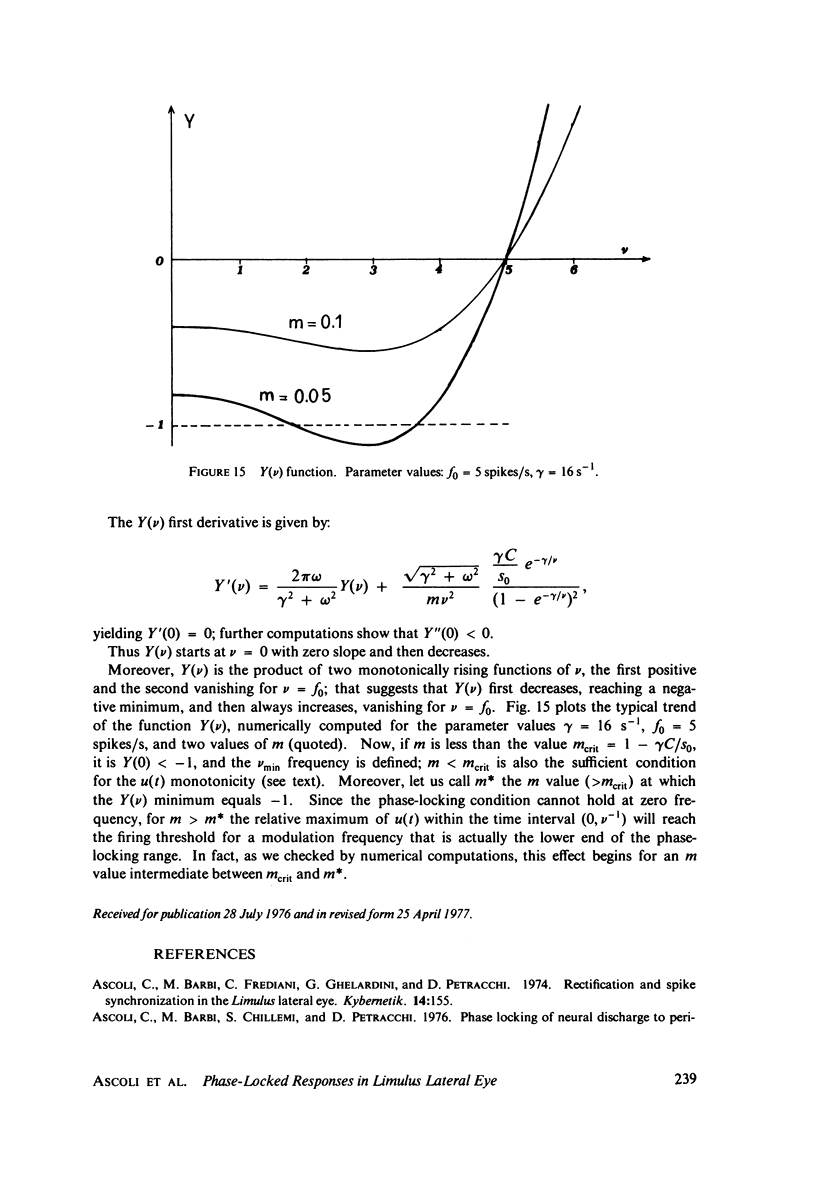
The 1:1 phase locking of the neural discharge to sinusoidally modulated stimuli was investigated both theoretically and experimentally. On the theoretical side, a neural encoder model, the self-inhibited leaky integrator, was considered, and the phase of the locked impulse was computed for each frequency in the locking range by imposing the condition that the "leaky integral" u(t) of the driving signal should reach the threshold for the first time one stimulus period after the preceding impulse. As u(t) can be a nonmonotonic function, this approach leads to results that sometimes differ from those reported in the literature. It turns out that the phase excursion is often much smaller than the values of about 180 degrees predicted from previous analysis. Moreover, our analysis shows a peculiar effect; the phase locking frequency range narrows when the input modulation depth increases. The theoretical predictions are then compared with phase-locked discharge patterns recorded from visual cells of the Limulus lateral eye, stimulated by sinusoidally modulated light or depolarizing current. The phases of the locked spikes at each of a number of modulation frequencies have been measured. The predictions offered by the model fit the experimental data, although there are some difficulties in determining the effective driving signal.
Full text
PDF





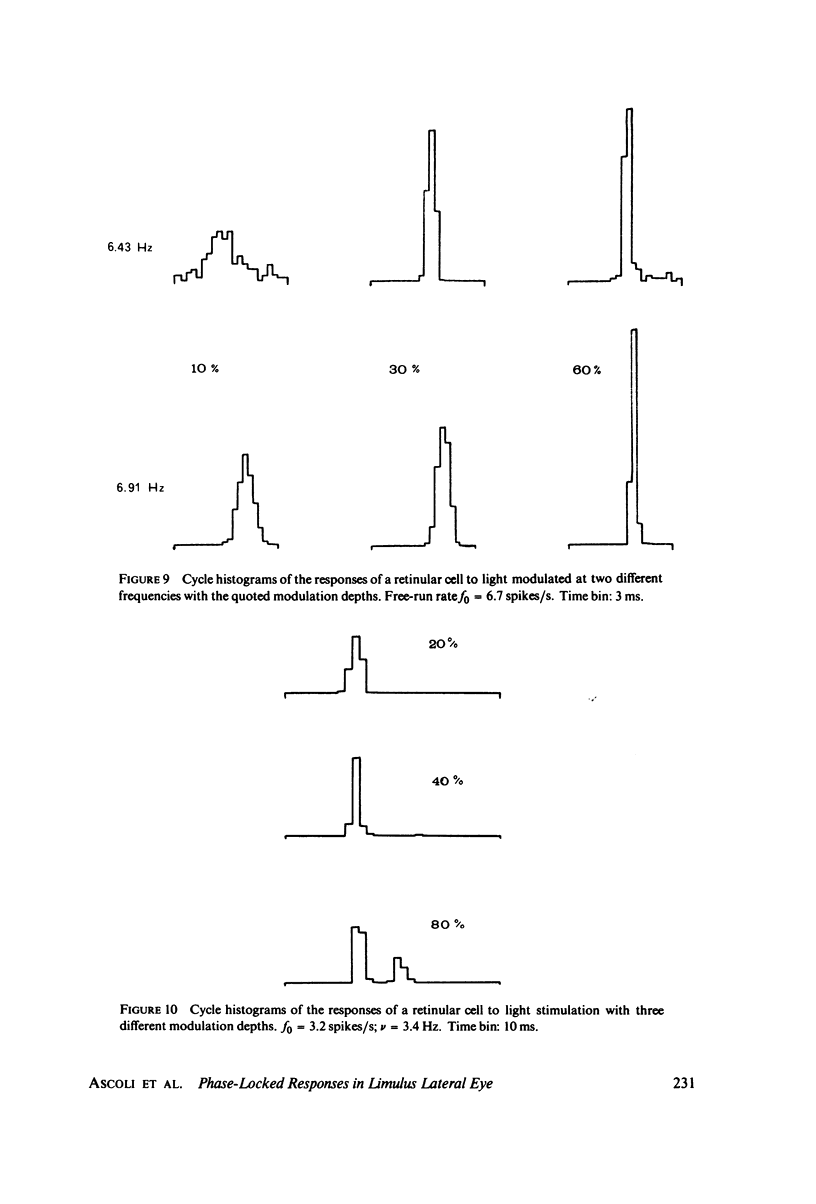
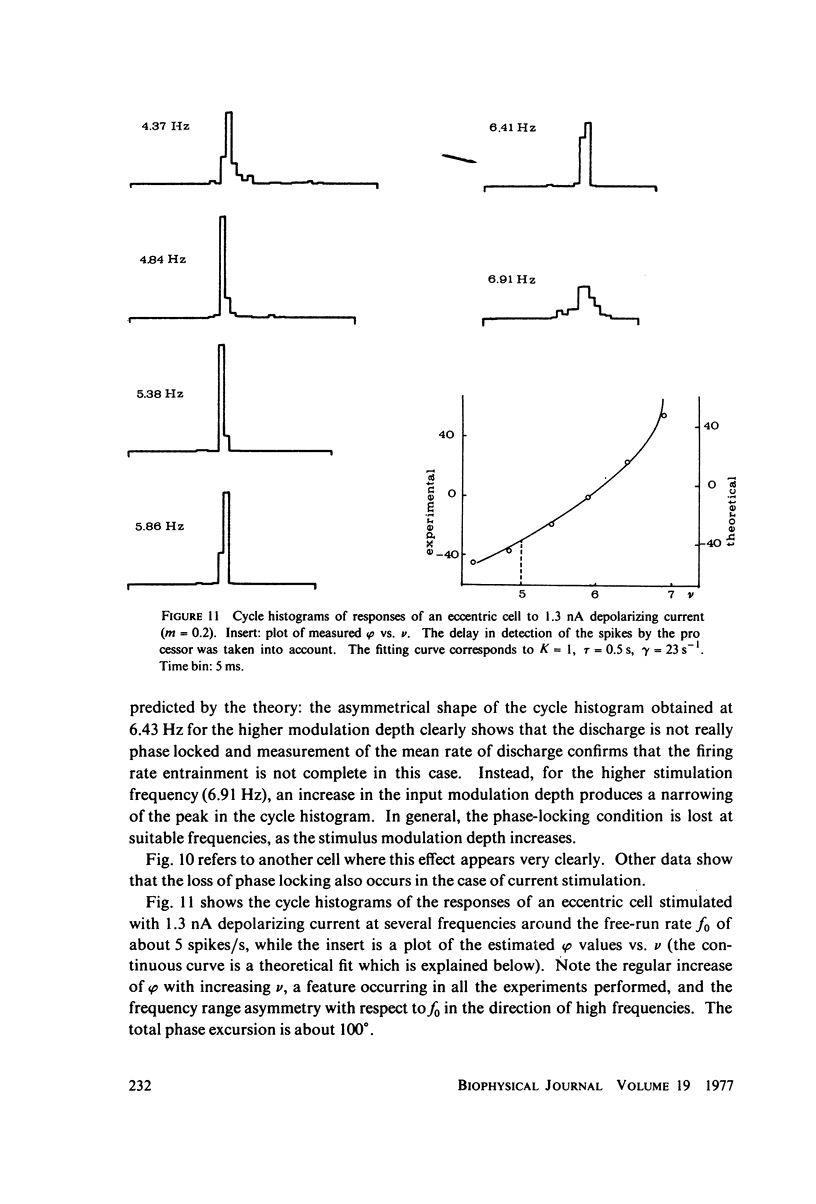
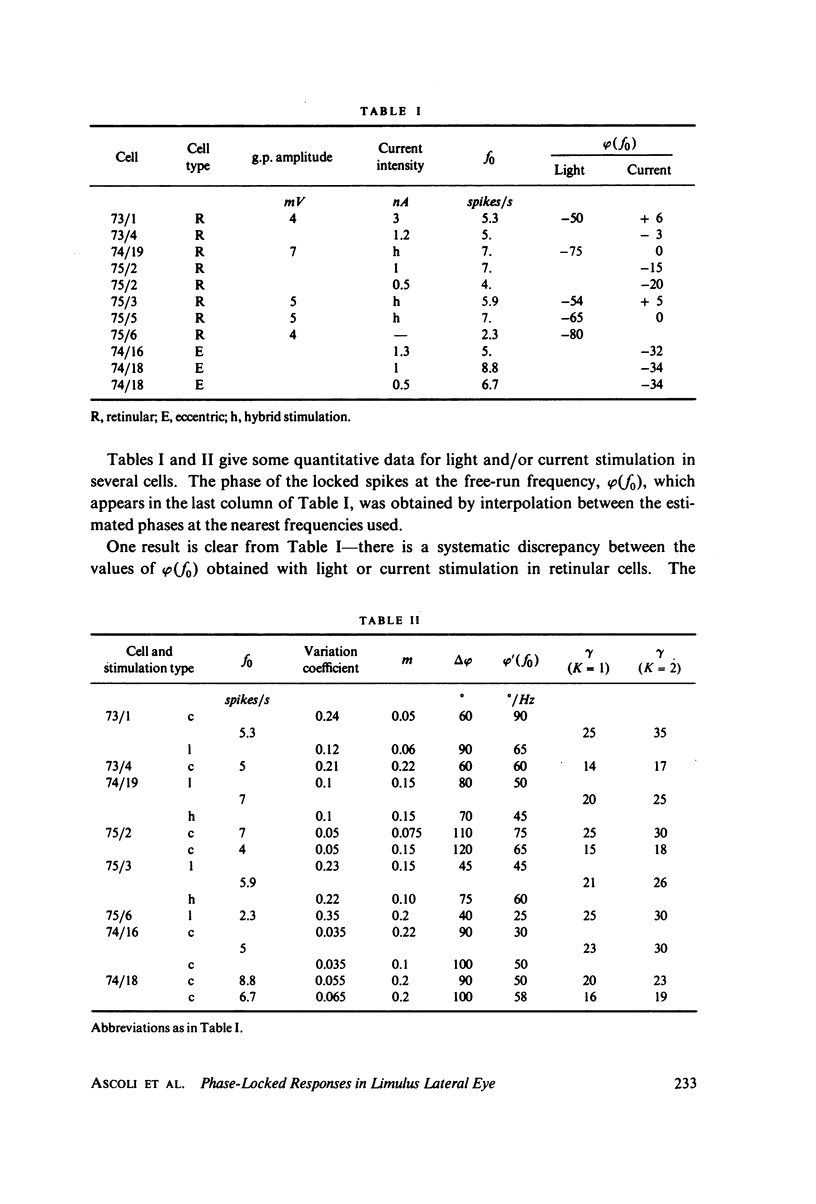
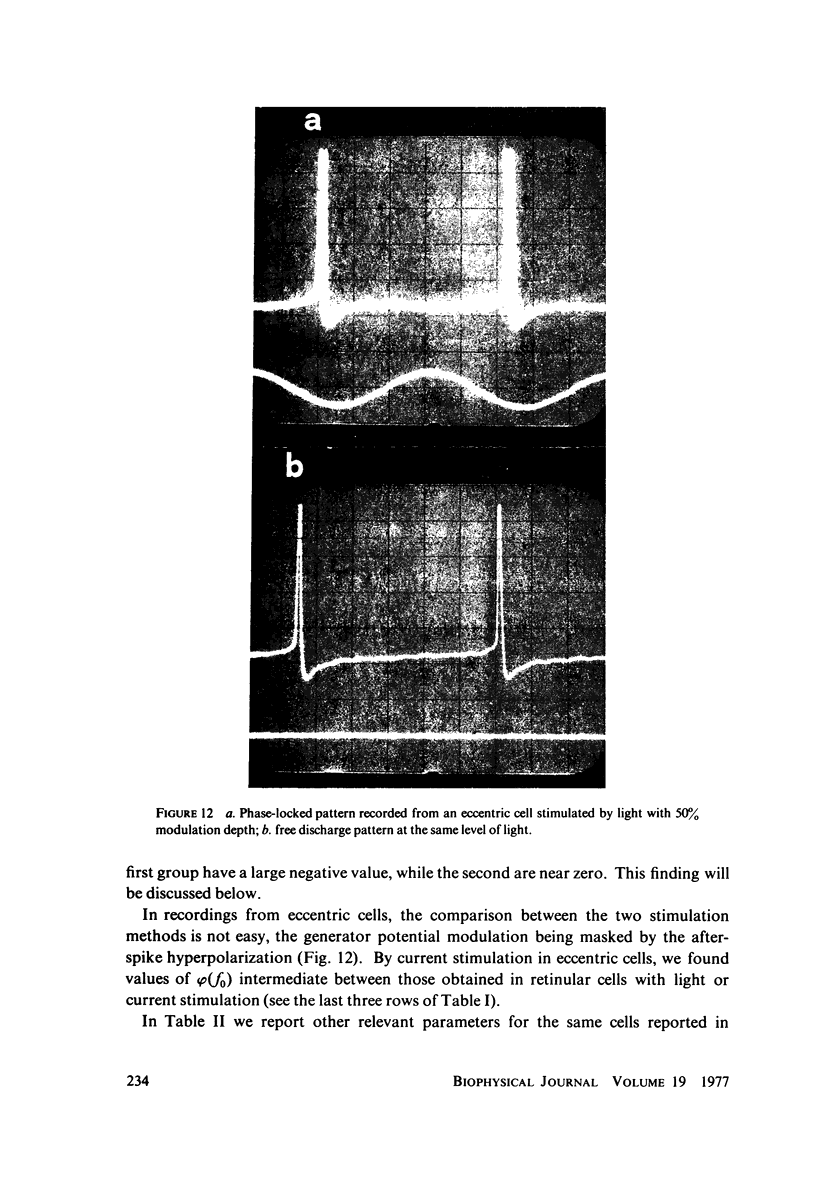



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli C., Barbi M., Frediani C., Ghelardini G., Petracchi D. Rectification and spike synchronization in the Limulus lateral eye. Kybernetik. 1974 Mar 13;14(3):155–160. doi: 10.1007/BF00288917. [DOI] [PubMed] [Google Scholar]
- Barbi M., Carelli V., Frediani C., Petracchi D. The self-inhibited leaky integrator: transfer functions and steady state relations. Biol Cybern. 1975 Oct 1;20(1):51–59. doi: 10.1007/BF00350999. [DOI] [PubMed] [Google Scholar]
- Bayly E. J. Spectral analysis of pulse frequency modulation in the nervous systems. IEEE Trans Biomed Eng. 1968 Oct;15(4):257–265. doi: 10.1109/tbme.1968.4502576. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., MANTEGAZZINI F. Interpretation of the repetitive firing of nerve cells. J Gen Physiol. 1962 Jul;45:1163–1179. doi: 10.1085/jgp.45.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister J. F., Poppele R. E., Purple R. L. Repetitive firing: dynamic behavior of sensory neurons reconciled with a quantitative model. J Neurophysiol. 1974 Nov;37(6):1213–1227. doi: 10.1152/jn.1974.37.6.1213. [DOI] [PubMed] [Google Scholar]
- Knight B. W. Dynamics of encoding in a population of neurons. J Gen Physiol. 1972 Jun;59(6):734–766. doi: 10.1085/jgp.59.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. W. The relationship between the firing rate of a single neuron and the level of activity in a population of neurons. Experimental evidence for resonant enhancement in the population response. J Gen Physiol. 1972 Jun;59(6):767–778. doi: 10.1085/jgp.59.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. W., Toyoda J. I., Dodge F. A., Jr A quantitative description of the dynamics of excitation and inhibition in the eye of Limulus. J Gen Physiol. 1970 Oct;56(4):421–437. doi: 10.1085/jgp.56.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppele R. E., Bowman R. J. Quantitative description of linear behavior of mammalian muscle spindles. J Neurophysiol. 1970 Jan;33(1):59–72. doi: 10.1152/jn.1970.33.1.59. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Chen W. J. Repetitive firing behavior of mammalian muscle spindle. J Neurophysiol. 1972 May;35(3):357–364. doi: 10.1152/jn.1972.35.3.357. [DOI] [PubMed] [Google Scholar]
- Purple R. L., Dodge F. A. Interaction of excitation and inhibition in the eccentric cell in the eye of Limulus. Cold Spring Harb Symp Quant Biol. 1965;30:529–537. doi: 10.1101/sqb.1965.030.01.051. [DOI] [PubMed] [Google Scholar]
- Rescigno A., Stein R. B., Purple R. L., Poppele R. E. A neuronal model for the discharge patterns produced by cyclic inputs. Bull Math Biophys. 1970 Sep;32(3):337–353. doi: 10.1007/BF02476873. [DOI] [PubMed] [Google Scholar]
- Smith T. G., Baumann F. The functional organization within the ommatidium of the lateral eye of limulus. Prog Brain Res. 1969;31:313–349. doi: 10.1016/S0079-6123(08)63248-3. [DOI] [PubMed] [Google Scholar]
- Stein R. B., French A. S., Holden A. V. The frequency response, coherence, and information capacity of two neuronal models. Biophys J. 1972 Mar;12(3):295–322. doi: 10.1016/S0006-3495(72)86087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA T. Peripheral mechanism of nervous activity in lateral eye of horseshoe crab. J Neurophysiol. 1957 May;20(3):245–254. doi: 10.1152/jn.1957.20.3.245. [DOI] [PubMed] [Google Scholar]