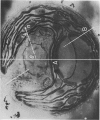
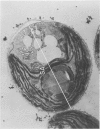
Abstract
Many cells and cell fragments are known to assume specific alignments with respect to an applied magnetic field. One indicator of this alignment is a difference between the intensities of fluorescence observed in polarizations parallel and perpendicular to the magnetic filed. We calculate these two intensities using a model that assumes axially symmetric membranes and that covers a wide variety of shapes from flat disk to right cylinder. The fluorescence is assumed to originate at chromophores randomly exicted but nonrandomly oriented in the membranes. The membrane alignment is assumed to be due to the net torque on a nonrandom distribution of diamagnetically anisotropic molecules. The predicted results are consistent with most magnetoorientation data from green cells, but we are able to show that Chlorella data are not consistent with the hypothesis that the membranes have, and maintain, a cuplike configuration.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Steele R., Mueller H. ON THE MAGNETIC ASYMMETRY OF MUSCLE FIBERS. Proc Natl Acad Sci U S A. 1958 Jan;44(1):1–4. doi: 10.1073/pnas.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J. F., Geacintov N. E., Van Nostrand F., Van Metter R. Orientation of chlorophyll in vivo. Studies with magnetic field oriented chlorella. Biochem Biophys Res Commun. 1973 Apr 2;51(3):597–602. doi: 10.1016/0006-291x(73)91356-9. [DOI] [PubMed] [Google Scholar]
- Becker J. F., Trentacosti F., Geacintov N. E. A linear dichroism study of the orientation of aromatic protein residues in magnetically oriented bovine rod outer segments. Photochem Photobiol. 1978 Jan;27(1):51–54. doi: 10.1111/j.1751-1097.1978.tb07564.x. [DOI] [PubMed] [Google Scholar]
- Breton J., Michel-Villaz M., Paillotin G. Orientation of pigments and structural proteins in the photosynthetic membrane of spinach chloroplasts: a linear dichroism study. Biochim Biophys Acta. 1973 Jul 26;314(1):42–56. doi: 10.1016/0005-2728(73)90062-5. [DOI] [PubMed] [Google Scholar]
- Chalazonitis N., Chagneux R., Arvanitaki A. Rotation des segments externes des photorécepeurs dans le champ magnétique constant. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jul 6;271(1):130–133. [PubMed] [Google Scholar]
- Clement-Metral J. D. Direct observation of the rotation in a constant magnetic field of highly organized lamellar structures. FEBS Lett. 1975 Feb 1;50(2):257–260. doi: 10.1016/0014-5793(75)80502-3. [DOI] [PubMed] [Google Scholar]
- GOEDHEER J. C. Orientation of the pigment molecules in the chloroplast. Biochim Biophys Acta. 1955 Apr;16(4):471–476. doi: 10.1016/0006-3002(55)90265-1. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., Van Norstrand F., Becker J. F., Tinkel J. B. Magnetic field induced orientation of photosynthetic systems. Biochim Biophys Acta. 1972 Apr 20;267(1):65–79. doi: 10.1016/0005-2728(72)90138-7. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., van Nostrand F., Becker J. F. Polarized light spectroscopy of photosynthetic membranes in magneto-oriented whole cells and chloroplasts. Fluorescence and dichroism. Biochim Biophys Acta. 1974 Jun 28;347(3):443–463. doi: 10.1016/0005-2728(74)90082-6. [DOI] [PubMed] [Google Scholar]
- Hoff A. J. Kinetics of populating and depopulating of the components of the photoinduced triplet state of the photosynthetic bacteria Rhodospirillum rubrum, Rhodopseudomonas spheroides (wild type), and its mutant R-26 as measured by ESR in zero-field. Biochim Biophys Acta. 1976 Sep 13;440(3):765–771. doi: 10.1016/0005-2728(76)90059-1. [DOI] [PubMed] [Google Scholar]
- Hoff A. J., Rademaker H., van Grondelle R., Duysens L. N. On the magnetic field dependence of the yield of the triplet state in reaction centers of photosynthetic bacteria. Biochim Biophys Acta. 1977 Jun 9;460(3):547–554. doi: 10.1016/0005-2728(77)90094-9. [DOI] [PubMed] [Google Scholar]
- Hong F. T., Mauzerall D., Mauro A. Magnetic anisotropy and the orientation of retinal rods in a homogeneous magnetic field. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1283–1285. doi: 10.1073/pnas.68.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Villaz M. Fluorescence polarization: pigment orientation and energy transfer in photosynthetic membranes. J Theor Biol. 1976 May 7;58(1):113–129. doi: 10.1016/0022-5193(76)90142-9. [DOI] [PubMed] [Google Scholar]
- Neugebauer D. C., Blaurock A. E. Magnetic orientation of purple membranes demonstrated by optical measurements and neutron scattering. FEBS Lett. 1977;78(1):31–35. doi: 10.1016/0014-5793(77)80266-4. [DOI] [PubMed] [Google Scholar]
- Paillotin G., Breton J. Orientation of chlorophylls within chloroplasts as shown by optical and electrochromic properties of the photosynthetic membrane. Biophys J. 1977 Apr;18(1):63–79. doi: 10.1016/S0006-3495(77)85597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler D. M. X-ray diffraction from chloroplast membranes oriented in a magentic field. FEBS Lett. 1976 Sep 1;67(3):289–293. doi: 10.1016/0014-5793(76)80549-2. [DOI] [PubMed] [Google Scholar]
- Swenberg C. E., Geacintov N. E., Pope M. Bimolecular quenching of excitons and fluorescence in the photosynthetic unit. Biophys J. 1976 Dec;16(12):1447–1452. doi: 10.1016/S0006-3495(76)85786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Metter R. L. Excitation energy transfer in the light-harvesting chlorophyll a/b.protein. Biochim Biophys Acta. 1977 Dec 23;462(3):642–658. doi: 10.1016/0005-2728(77)90107-4. [DOI] [PubMed] [Google Scholar]