Abstract
Photosynthetic application of picosecond spectroscopic techniques to bacterial reaction centers has led to a much greater understanding of the chemical nature of the initial steps of photosynthesis. Within 10 ps after excitation, a charge transfer complex is formed between the primary donor, a “special pair” of bacteriochlorophyll molecules, and a transient acceptor involving bacteriopheophytin. This complex subsequently decays in about 120 ps by donating the electron to a metastable acceptor, a tightly bound quinone.
Recent experiments with conventional optical and ESR techniques have shown that when reaction centers are illuminated by a series of single turnover flashes in the presence of excess electron donors and acceptors, a stable, anionic ubisemiquinone is formed on odd flashes and destroyed on even flashes, suggesting that the acceptor region contains a second quinone that acts as a two-electron gate between the reaction center and subsequent electron transport events involving the quinone pool.
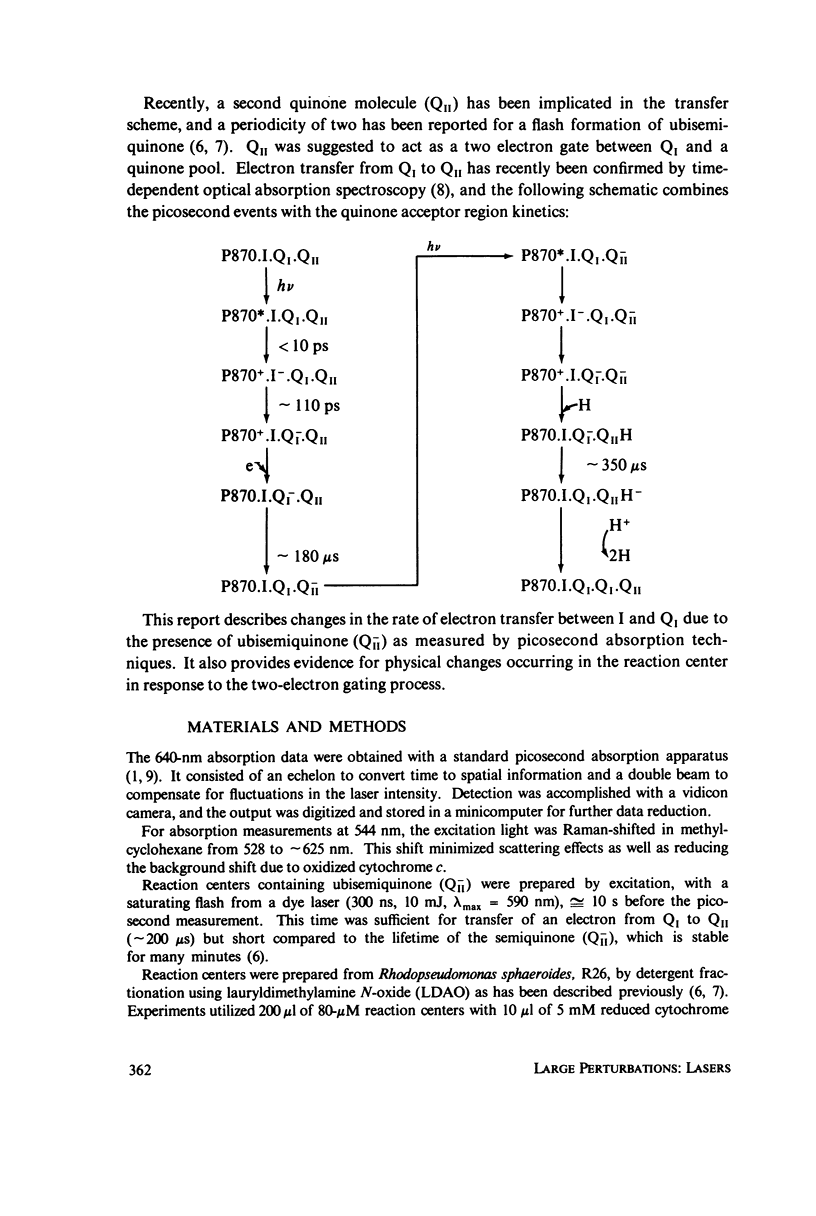
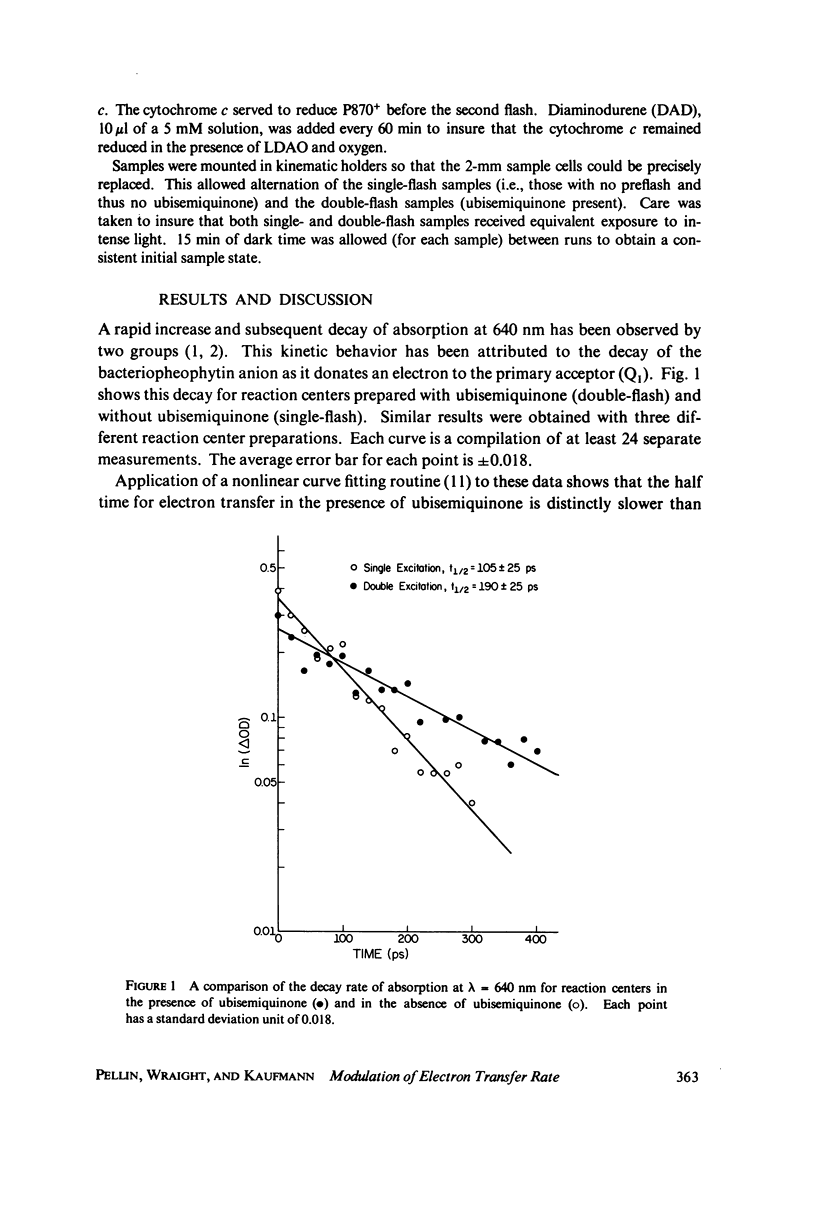
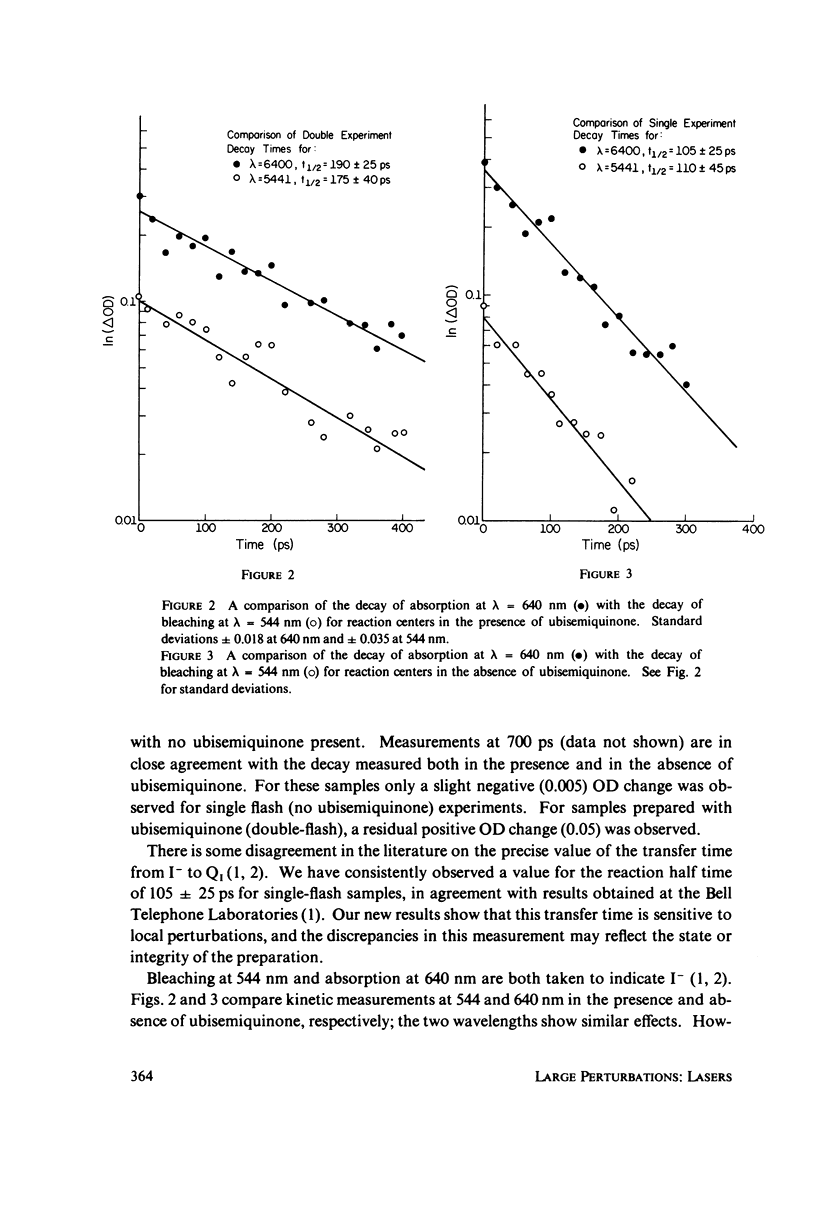
Utilizing standard picosecond techniques, we have examined the decay of the charge transfer complex in reaction centers in the presence of the stable semiquinone, formed by flash illumination with a dye laser 10 s before excitation by a picosecond pulse. In this state the decay rate for the charge transfer complex is considerably slower than when no electron is present in the quinone acceptor region. This indicates fairly strong coupling between constituents of the reaction center-quinone acceptor complex and may provide a probe into the relative positions of the various components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch G. E., Rentzepis P. M. Picosecond chemistry. Science. 1976 Oct 15;194(4262):276–283. doi: 10.1126/science.968481. [DOI] [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Kaufmann K. J., Chance B., Rentzepis P. M. Picosecond kinetics of the 1250 nm band of the Rps. sphaeroides reaction center: the nature of the primary photochemical intermediary state. FEBS Lett. 1975 Dec 15;60(2):275–280. doi: 10.1016/0014-5793(75)80730-7. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Photo-induced charge transfer. A critical test of the mechanism and range of biological electron transfer processes. Biophys J. 1977 Jun;18(3):311–321. doi: 10.1016/S0006-3495(77)85616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Vermeglio A., Clayton R. K. Kinetics of electron transfer between the primary and the secondary electron acceptor in reaction centers from Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1977 Jul 7;461(1):159–165. doi: 10.1016/0005-2728(77)90078-0. [DOI] [PubMed] [Google Scholar]
- Vermeglio A. Secondary electron transfer in reaction centers of Rhodopseudomonas sphaeroides. Out-of-phase periodicity of two for the formation of ubisemiquinone and fully reduced ubiquinone. Biochim Biophys Acta. 1977 Mar 11;459(3):516–524. doi: 10.1016/0005-2728(77)90050-0. [DOI] [PubMed] [Google Scholar]
- Wraight C. A. Electron acceptors of photosynthetic bacterial reaction centers. Direct observation of oscillatory behaviour suggesting two closely equivalent ubiquinones. Biochim Biophys Acta. 1977 Mar 11;459(3):525–531. doi: 10.1016/0005-2728(77)90051-2. [DOI] [PubMed] [Google Scholar]


