Table 12.
Association of Psoriasis with Haplotypes Carrying a Recombination between the Risk Alleles of HLA-C and CDSN
| TDT |
PDT |
||||||||||
|
P |
Power |
P |
|||||||||
|
Recombinant Haplotype and Test Regiona |
Map Bounds (kb)b | Haplotype Frequencyc | T:NT (%T)e | Uncorrectedf | Correctedg | Uncorrectedh | Correctedi |
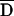 j j
|
Uncorrectedf | Correctedg | Excluded as Risk?d |
| HLA-Cw6+/CDSN*TTC−: | |||||||||||
| CDSN | 590.5–595.4 | .0056 | 11:2 (84.6) | .023 | .076 | … | … | .643 | .0042 | .014 | Yes |
| M6S190–M6S224 | 583.3–658.1 | .0049 | 9:1 (90.0) | .022 | .071 | … | … | .667 | .0074 | .029 | Yes |
| M6S111–M6S224 | 528.9–658.1 | .0028 | 5:0 (100.0) | .033 | .19 | … | … | .750 | .037 | .18 | No |
| HLA-Cw6−/CDSN*TTC+: | |||||||||||
| CDSN | 590.5–595.4 | .0209 | 15:26 (36.6) | .12 | .33 | .995 | .983 | −.248 | .19 | .70 | Yes |
| CDSN-M6S162 | 590.5–655.7 | .0122 | 9:18 (33.3) | .12 | .34 | .938 | .861 | −.295 | .22 | .77 | No |
| CDSN-M6S224 | 590.5–658.1 | .0066 | 5:9 (35.7) | .42 | .86 | .700 | .527 | −.283 | .39 | .95 | No |
| M6S190-CDSN | 583.3–595.4 | .0199 | 15:24 (38.5) | .20 | .52 | .994 | .980 | −.212 | .36 | .93 | Yes |
| M6S198-CDSN | 566.7–595.4 | .0105 | 8:13 (38.1) | .38 | .81 | .899 | .790 | −.261 | .42 | .96 | No |
| M6S200-CDSN | 561.7–595.4 | .0063 | 5:8 (38.5) | .58 | .95 | .677 | .498 | −.214 | .66 | 1.00 | No |
| HCR-CDSN | 551.1–595.4 | .0049 | 4:7 (36.4) | .55 | .94 | .553 | .363 | −.250 | .62 | .97 | No |
Bounds of the test region with use of the markers and genes of figure 2. To be conservative, the smallest possible portion of the extended HLA-Cw6+/CDSN*TTC+ risk haplotype not carried by HLA-Cw6+/CDSN*TTC− recombinant haplotypes and the smallest possible portion of the extended risk haplotype carried by HLA-Cw6−/CDSN*TTC+ recombinant haplotypes were used to determine whether a recombinant haplotype qualified for assessing the exclusion of the test region from the PSORS1 candidate interval.
Map bounds refer to the map coordinate system of figure 2.
Frequency of HLA-Cw6+/CDSN*TTC− recombinant haplotypes not carrying the test region or the frequency of HLA-Cw6−/CDSN*TTC+ recombinants carrying the test region; results are based on 2,867 founder chromosomes in 678 pedigrees.
The test region is considered excluded from the PSORS1 candidate interval if the corrected TDT or PDT P value is <.05 for the positively associated HLA-Cw6+/CDSN*TTC− haplotypes or if the corrected TDT power was at least 95% for the unassociated HLA-Cw6−/CDSN*TTC+ haplotypes.
For the biallelic TDT.
Nominal P value uncorrected for multiple testing.
P value corrected for multiple testing (see the “Subjects and Methods” section for details of correction procedure).
Nominal power of the TDT on the basis of a type I error rate of 0.05, an additive model with a GRR2 of 5, and a disease prevalence of 0.02.
Corrected power of the TDT based on a type I error rate of 0.016, which ensures an experimentwide type I error rate of 0.05 for all tests in the table, an additive model with a GRR2 of 5, and a disease prevalence of 0.02.
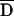 is a standardized measure of disequilibrium for the PDT (see the “Subjects and Methods” section).
is a standardized measure of disequilibrium for the PDT (see the “Subjects and Methods” section).
