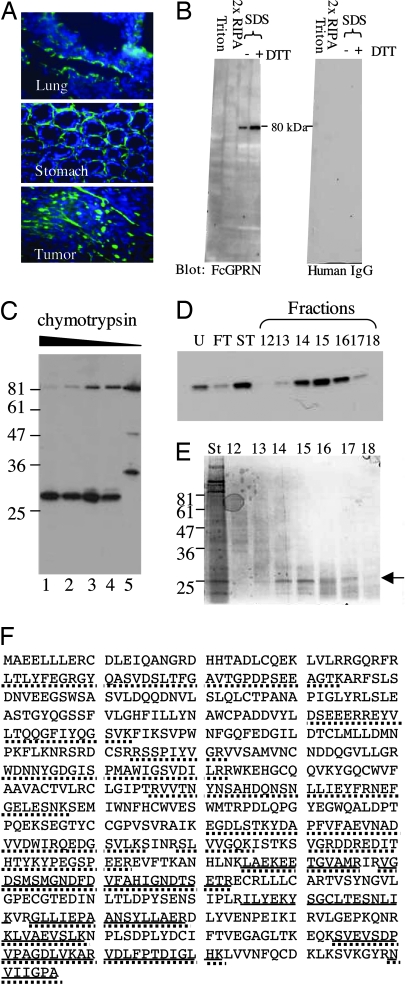
Fig. 3.
Identification of TG2 as a candidate GPR56-binding protein. (A) The N terminus of GPR56 that was fused to human Fc fragment (FcGPRN) recognizes extracellular matrix on tissue and tumor sections by immunohistochemistry. Blue, DAPI; green, FcGPRN. (B) FcGPRN recognized a band of ≈80 kDa in the radioimmunoprecipitation assay (RIPA)-insoluble fraction of lung lysate. The extraction scheme is shown in Fig. 9. Human IgG was used as a negative control. (C) FcGPRN recognizes an ≈28-kDa fragment after chymotryptic digestion of mouse lungs. Lanes 1–4 are 1:100, 1:250, 1:500, and 1:1,000 dilutions of 2.5% chymotrypsin. Lane 5 is a negative control with no chymotrypsin treatment. (D) The fractions after purification (indicated in Fig. 10) were probed by FcGPRN on a Western blot. (E) Some of the samples shown in D were stained with SimplyBlue stain and the ≈28-kDa bands were excised for mass spectrometric analysis (arrow). (F) Identification of mouse TG2 as a candidate protein from mass spectrometric analysis. The sequence of TG2 protein is shown. Bold lines indicate the sequences found in the ≈28-kDa fragment, and the dotted lines indicate the sequences found in the uncleaved ≈80-kDa protein. The ≈28-kDa fragment is located at the C terminus of the protein.