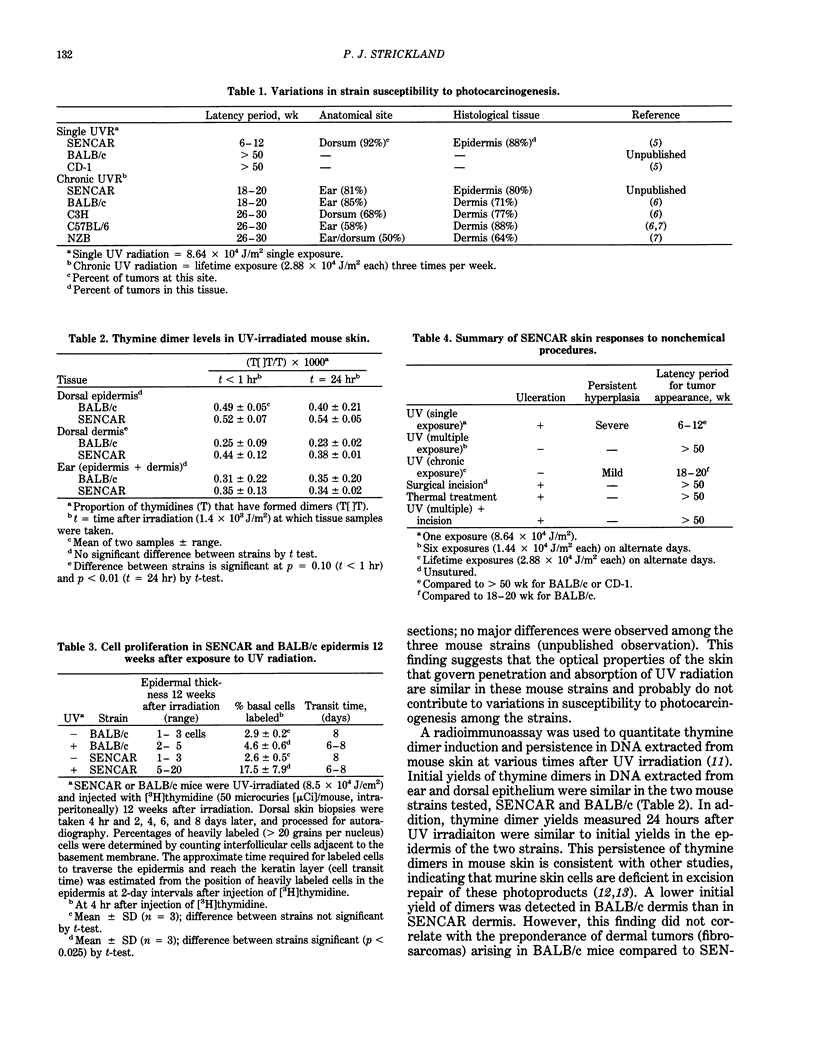
Abstract
Susceptibility to photocarcinogenesis has been examined in several mouse strains and stocks including SENCAR, CD-1, BALB/c, C3H, C57Bl, and NZB, SENCAR mice are hypersusceptible to tumorigenesis caused by single high dose exposures to ultraviolet (UV) radiation but not by chronic low-dose exposures. SENCAR mice also exhibit an exaggerated and persistent epidermal hyperplasia in response to UV-induced tissue damage. The persistent hyperplasia is apparently due to a sustained proliferation of the epithelial basal cells, rather than to delayed cell differentiation. SENCAR mice did not exhibit persistent hyperplasia following other forms of tissue damage (surgical or thermal). In related studies, the levels of thymine dimers induced in SENCAR epidermis by UV radiation were comparable to those observed in BALB/c epidermis. In addition, no differences were found in the tissue distribution or persistence of thymine dimers in SENCAR and BALB/c skin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUTWELL R. K. SOME BIOLOGICAL ASPECTS OF SKIN CARCINOGENISIS. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J., Slaga T. J., Boutwell R. K. Comparison of the tumor-initiating activity of 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene in female SENCAR and CS-1 mice. Carcinogenesis. 1980 May;1(5):381–389. doi: 10.1093/carcin/1.5.381. [DOI] [PubMed] [Google Scholar]
- Hennings H., Devor D., Wenk M. L., Slaga T. J., Former B., Colburn N. H., Bowden G. T., Elgjo K., Yuspa S. H. Comparison of two-stage epidermal carcinogenesis initiated by 7,12-dimethylbenz(a)anthracene or N-methyl-N'-nitro-N-nitrosoguanidine in newborn and adult SENCAR and BALB/c mice. Cancer Res. 1981 Mar;41(3):773–779. [PubMed] [Google Scholar]
- Johnson B. E. Formation of thymine containing dimers in skin exposed to ultraviolet radiation. Bull Cancer. 1978;65(3):283–297. [PubMed] [Google Scholar]
- Kripke M. L. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977 May;37(5):1395–1400. [PubMed] [Google Scholar]
- Regan J. D., Carrier W. L., Smith D. P., Waters R., Lee W. H. Pyrimidine dimer excision in human cells and skin cancer. Natl Cancer Inst Monogr. 1978 Dec;(50):141–143. [PubMed] [Google Scholar]
- Strickland J. E., Strickland A. G. Host cell reactivation studies with epidermal cells of mice sensitive and resistant to carcinogenesis. Cancer Res. 1984 Mar;44(3):893–895. [PubMed] [Google Scholar]
- Strickland P. T. Abnormal wound healing in UV-irradiated skin of Sencar mice. J Invest Dermatol. 1986 Jan;86(1):37–41. doi: 10.1111/1523-1747.ep12283779. [DOI] [PubMed] [Google Scholar]
- Strickland P. T., Boyle J. M. Characterisation of two monoclonal antibodies specific for dimerised and non-dimerised adjacent thymidines in single stranded DNA. Photochem Photobiol. 1981 Nov;34(5):595–601. [PubMed] [Google Scholar]
- Strickland P. T. Photocarcinogenesis and influence of UV radiation on autoimmune disease in NZB/N mice. J Natl Cancer Inst. 1984 Aug;73(2):537–541. doi: 10.1093/jnci/73.2.537. [DOI] [PubMed] [Google Scholar]
- Strickland P. T. Tumor induction in Sencar mice in response to ultraviolet radiation. Carcinogenesis. 1982;3(12):1487–1489. doi: 10.1093/carcin/3.12.1487. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Morgan D. L. Mouse skin cells resistant to terminal differentiation associated with initiation of carcinogenesis. Nature. 1981 Sep 3;293(5827):72–74. doi: 10.1038/293072a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Spangler E. F., Donahoe R., Geusz S., Ferguson E., Wenk M., Hennings H. Sensitivity to two-stage carcinogenesis of SENCAR mouse skin grafted to nude mice. Cancer Res. 1982 Feb;42(2):437–439. [PubMed] [Google Scholar]


