Abstract
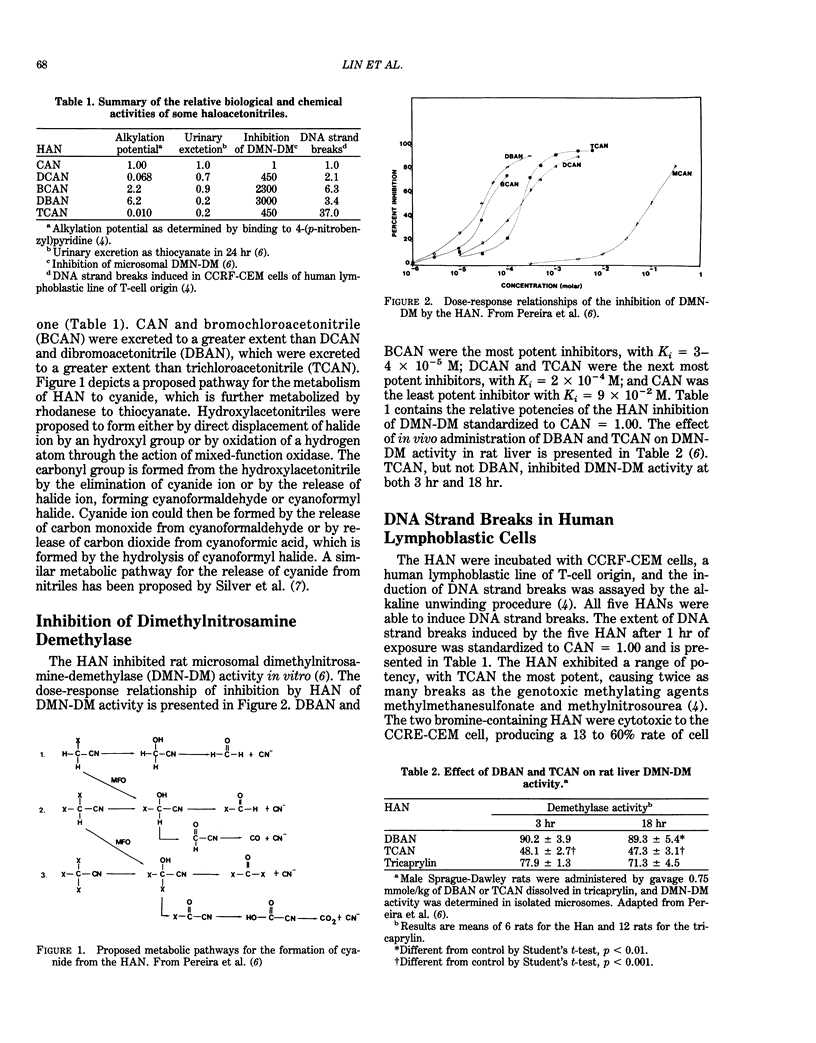
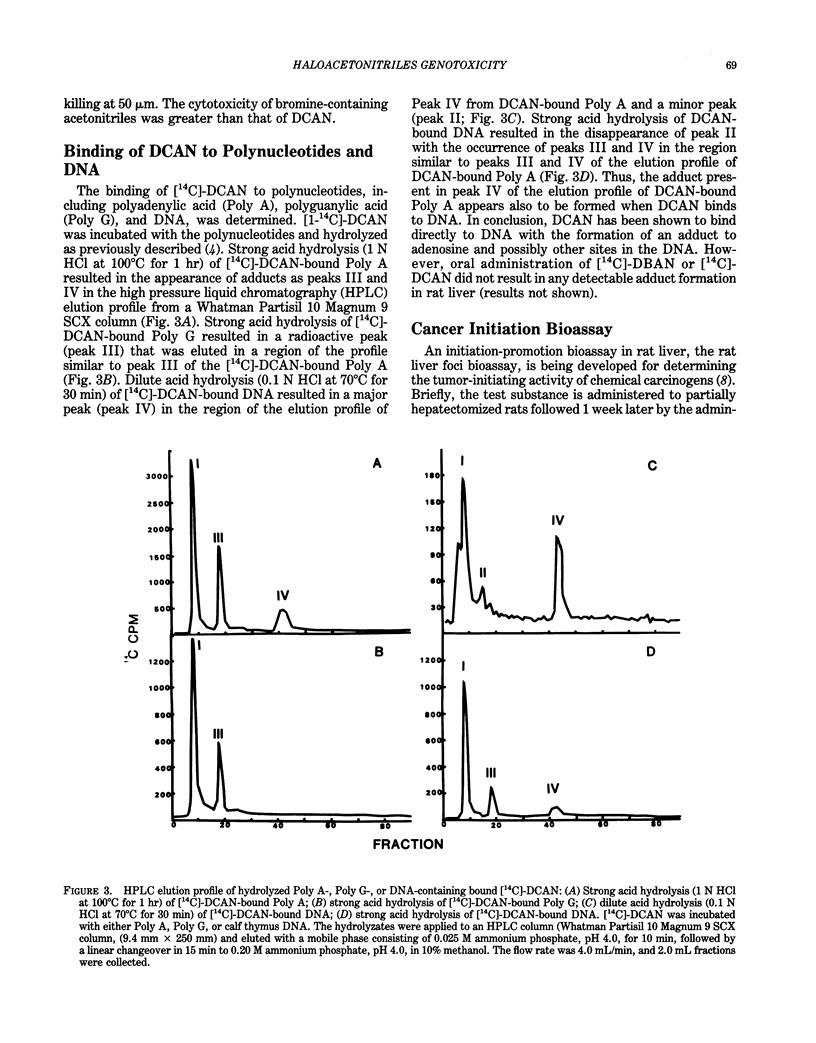
Haloacetonitriles (HAN) are drinking water contaminants produced during chlorine disinfection. This paper evaluates metabolism, genotoxicity, and tumor-initiating activity of these chemicals. The alkylating potential of the HAN to react with the electrophile-trapping agent, 4-(p-nitrobenzyl)pyridine, followed the order dibromoacetonitrile (DBAN) greater than bromochloroacetonitrile (BCAN) greater than chloroacetonitrile (CAN) greater than dichloroacetonitrile (DCAN) greater than trichloroacetonitrile (TCAN). When administered orally to rats, the HAN were metabolized to cyanide and excreted in the urine as thiocyanate. The extent of thiocyanate excretion was CAN greater than BCAN greater than DCAN greater than DBAN much greater than TCAN. Haloacetonitriles inhibited in vitro microsomal dimethylnitrosamine demethylase (DMN-DM) activity. The most potent inhibitors were DBAN and BCAN, with Ki = 3-4 X 10(-5) M; the next potent were DCAN and TCAN, with Ki = 2 X 10(-4) M; and the least potent inhibitor was CAN, with Ki = 9 X 10(-2) M. When administered orally, TCAN, but not DBAN, inhibited hepatic DMN-DM activity. The HAN produced DNA strand breaks in cultured human lymphoblastic (CCRF-CEM) cells. TCAN was the most potent DNA strand breaker, and BCAN greater than DBAN greater than DCAN greater than CAN, which was only marginally active. DCAN reacted with polyadenylic acid and DNA to form adducts in a cell-free system; however, the oral administration of DBAN or DCAN to rats did not result in detectable adduct formation in liver DNA. None of the HAN initiated gamma-glutamyltranspeptidase (GGT) foci when assayed for tumor-initiating activity in rat liver foci bioassay.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bull R. J., Meier J. R., Robinson M., Ringhand H. P., Laurie R. D., Stober J. A. Evaluation of mutagenic and carcinogenic properties of brominated and chlorinated acetonitriles: by-products of chlorination. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 1):1065–1074. doi: 10.1016/0272-0590(85)90142-3. [DOI] [PubMed] [Google Scholar]
- Craddock V. M., Frei J. V. Induction of liver cell adenomata in the rat by a single treatment with N-methyl-N-nitrosourea given at various times after partial hepatectomy. Br J Cancer. 1974 Dec;30(6):503–511. doi: 10.1038/bjc.1974.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel F. B., Schenck K. M., Mattox J. K., Lin E. L., Haas D. L., Pereira M. A. Genotoxic properties of haloacetonitriles: drinking water by-products of chlorine disinfection. Fundam Appl Toxicol. 1986 Apr;6(3):447–453. doi: 10.1016/0272-0590(86)90218-6. [DOI] [PubMed] [Google Scholar]
- Farber E. The sequential analysis of liver cancer induction. Biochim Biophys Acta. 1980 May 6;605(2):149–166. doi: 10.1016/0304-419x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Herren-Freund S. L., Pereira M. A. Carcinogenicity of by-products of disinfection in mouse and rat liver. Environ Health Perspect. 1986 Nov;69:59–65. doi: 10.1289/ehp.866959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink F. L., Coleman W. E., Munch J. W., Kaylor W. H., Ringhand H. P. In vivo formation of halogenated reaction products following peroral sodium hypochlorite. Bull Environ Contam Toxicol. 1983 Apr;30(4):394–399. doi: 10.1007/BF01610150. [DOI] [PubMed] [Google Scholar]
- Pereira M. A., Herren-Freund S. L., Britt A. L., Khoury M. M. Effects of strain, sex, route of administration and partial hepatectomy on the induction by chemical carcinogens of gamma-glutamyltranspeptidase foci in rat liver. Cancer Lett. 1983 Sep;20(2):207–214. doi: 10.1016/0304-3835(83)90050-2. [DOI] [PubMed] [Google Scholar]
- Pereira M. A., Lin L. H., Mattox J. K. Haloacetonitrile excretion as thiocyanate and inhibition of dimethylnitrosamine demethylase: a proposed metabolic scheme. J Toxicol Environ Health. 1984;13(4-6):633–641. doi: 10.1080/15287398409530527. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta. 1980 May 6;605(2):191–215. doi: 10.1016/0304-419x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Silver E. H., Kuttab S. H., Hasan T., Hassan M. Structural considerations in the metabolism of nitriles to cyanide in vivo. Drug Metab Dispos. 1982 Sep-Oct;10(5):495–498. [PubMed] [Google Scholar]
- Tsuda H., Lee G., Farber E. Induction of resistant hepatocytes as a new principle for a possible short-term in vivo test for carcinogens. Cancer Res. 1980 Apr;40(4):1157–1164. [PubMed] [Google Scholar]


