Abstract
The gill epithelium is the site of gas exchange, ionic regulation, acid-base balance, and nitrogenous waste excretion by fishes. The last three processes are controlled by passive and active transport of various solutes across the epithelium. Various environmental pollutants (e.g., heavy metals, acid rain, and organic xenobiotics) have been found to affect the morphology of the gill epithelium. Associated with these morphological pathologies, one finds alterations in blood ionic levels, as well as gill Na,K-activated ATPase activity and ionic fluxes. Such physiological disturbances may underly the toxicities of these pollutants. In addition, the epithelial transport steps which are affected in the fish gill model resemble those described in the human gut and kidney, sites of action of a variety of environmental toxins.
Full text
PDF
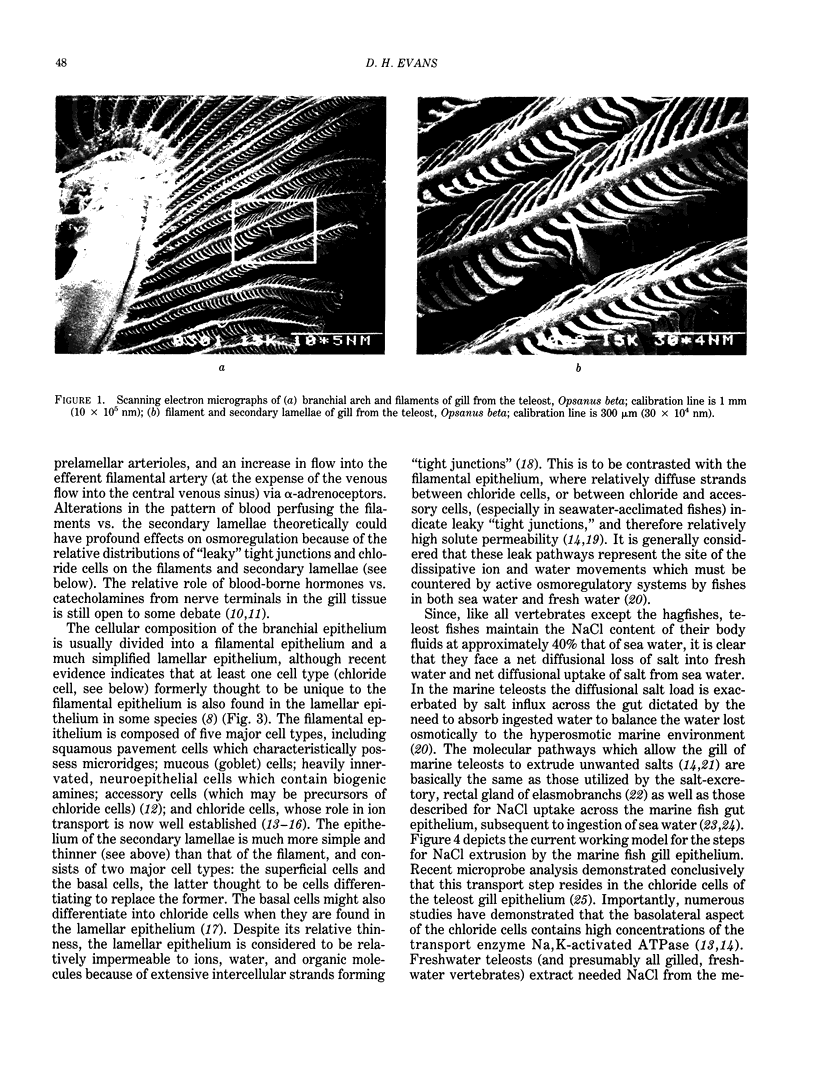
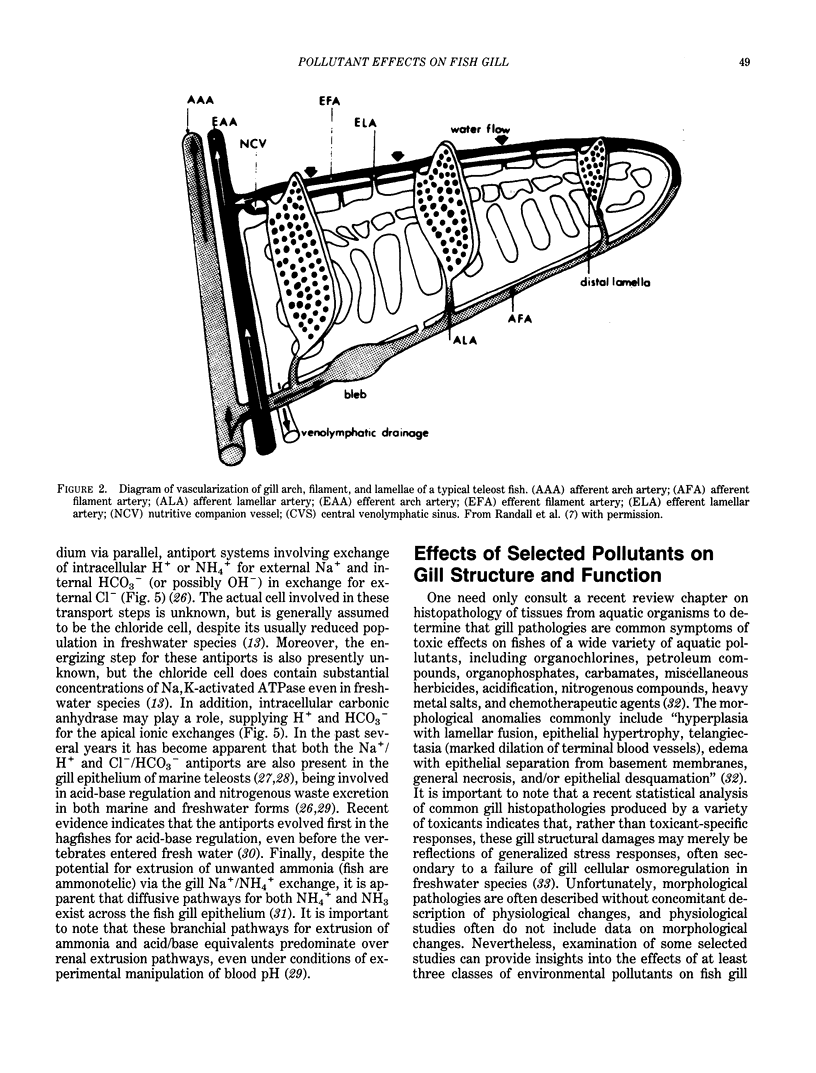
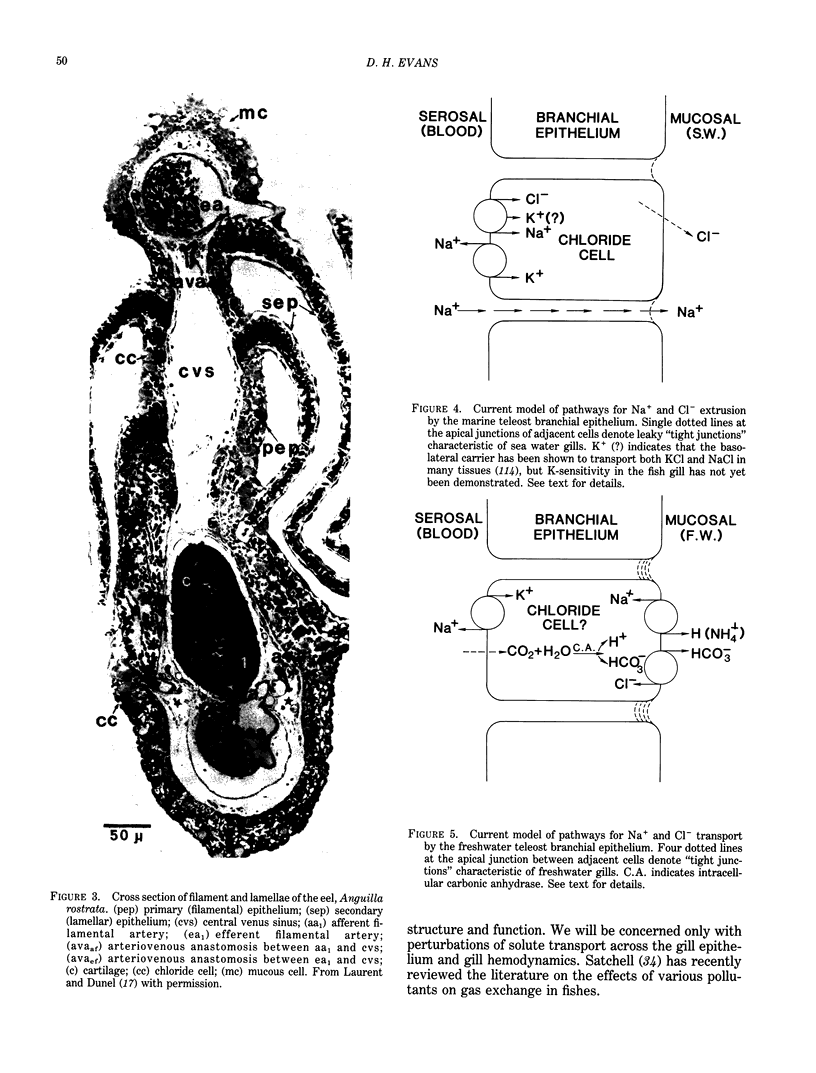








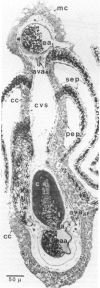
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Bornancin M., Cuthbert A. W., Maetz J. The effects of calcium on branchial sodium fluxes in the sea-water adapted eel, Anguilla anguilla, L. J Physiol. 1972 Apr;222(2):487–496. doi: 10.1113/jphysiol.1972.sp009810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J. C., Evans D. H. The role of environmental calcium in freshwater survival of the marine teleost, Lagodon rhomboides. J Exp Biol. 1976 Dec;65(3):529–538. doi: 10.1242/jeb.65.3.529. [DOI] [PubMed] [Google Scholar]
- Christensen G. M., Tucker J. H. Effects of selected water toxicants on the in vitro activity of fish carbonic anhydrase. Chem Biol Interact. 1976 May;13(2):181–192. doi: 10.1016/0009-2797(76)90007-7. [DOI] [PubMed] [Google Scholar]
- Claude P., Goodenough D. A. Fracture faces of zonulae occludentes from "tight" and "leaky" epithelia. J Cell Biol. 1973 Aug;58(2):390–400. doi: 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin D. A., Leray C. Interaction of adenosine and its phosphorylated derivatives with putative purinergic receptors in the gill vascular bed of rainbow trout. Pflugers Arch. 1979 Dec;383(1):35–40. doi: 10.1007/BF00584472. [DOI] [PubMed] [Google Scholar]
- Davis P. W., Wedemeyer G. A. Na + , K + -activated-ATPase inhibition in rainbow trout: a site for organochlorine pesticide toxicity? Comp Biochem Physiol B. 1971 Nov 15;40(3):823–827. doi: 10.1016/0305-0491(71)90157-x. [DOI] [PubMed] [Google Scholar]
- Daye P. G., Garside E. T. Histopathologic changes in surficial tissues of brook trout, Salvelinus fontinalis (Mitchill), exposed to acute and chronic levels of pH. Can J Zool. 1976 Dec;54(12):2140–2155. doi: 10.1139/z76-248. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Stoff J. S., Silva P. Mechanism and control of hyperosmotic NaCl-rich secretion by the rectal gland of Squalus acanthias. J Exp Biol. 1983 Sep;106:25–41. doi: 10.1242/jeb.106.1.25. [DOI] [PubMed] [Google Scholar]
- Evans D. H. Gill Na+/H+ and Cl-/HCO3- exchange systems evolved before the vertebrates entered fresh water. J Exp Biol. 1984 Nov;113:465–469. doi: 10.1242/jeb.113.1.465. [DOI] [PubMed] [Google Scholar]
- Field M., Karnaky K. J., Jr, Smith P. L., Bolton J. E., Kinter W. B. Ion transport across the isolated intestinal mucosa of the winter flounder, Pseudopleuronectes americanus. I. Functional and structural properties of cellular and paracellular pathways for Na and Cl. J Membr Biol. 1978 Jul 5;41(3):265–293. doi: 10.1007/BF01870433. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Bern H. A., Machen T. E., Conner M. Chloride cells and the hormonal control of teleost fish osmoregulation. J Exp Biol. 1983 Sep;106:255–281. doi: 10.1242/jeb.106.1.255. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Scheffey C. The chloride cell: definitive identification as the salt-secretory cell in teleosts. Science. 1982 Jan 8;215(4529):164–166. doi: 10.1126/science.7053566. [DOI] [PubMed] [Google Scholar]
- Hootman S. R., Philpott C. W. Accessory cells in teleost branchial epithelium. Am J Physiol. 1980 Mar;238(3):R199–R206. doi: 10.1152/ajpregu.1980.238.3.R199. [DOI] [PubMed] [Google Scholar]
- Hossler F. E., Musil G., Karnaky K. J., Jr, Epstein F. H. Surface ultrastructure of the gill arch of the killifish, Fundulus heteroclitus, from seawater and freshwater, with special reference to the morphology of apical crypts of chloride cells. J Morphol. 1985 Sep;185(3):377–386. doi: 10.1002/jmor.1051850309. [DOI] [PubMed] [Google Scholar]
- Janicki R. H., Kinter W. B. DDT inhibits Na + , K + , Mg 2+ -ATPase in the intestinal mucosae and gills of marine teleosts. Nat New Biol. 1971 Sep 29;233(39):148–149. doi: 10.1038/newbio233148a0. [DOI] [PubMed] [Google Scholar]
- Jha L. B., Bhatia B. Effect of mercuric chloride on coronary flow in perfused rat heart. Bull Environ Contam Toxicol. 1983 Aug;31(2):132–138. doi: 10.1007/BF01607883. [DOI] [PubMed] [Google Scholar]
- Kinter W. B., Merkens L. S., Janicki R. H., Guarino A. M. Studies on the Mechanism of Toxicity of DDT and Polychlorinated Biphenyls: Disruption of Osmoregulation in Marine Fish. Environ Health Perspect. 1972 Apr;1:169–173. doi: 10.1289/ehp.7201169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P., Dunel S. Morphology of gill epithelia in fish. Am J Physiol. 1980 Mar;238(3):R147–R159. doi: 10.1152/ajpregu.1980.238.3.R147. [DOI] [PubMed] [Google Scholar]
- Leadem T. P., Campbell R. D., Johnson D. W. Osmoregulatory responses to DDT and varying salinities in Salmo gairdneri. I. Gill Na-K-ATPase. Comp Biochem Physiol A Comp Physiol. 1974 Sep 1;49(1A):197–205. doi: 10.1016/0300-9629(74)90555-6. [DOI] [PubMed] [Google Scholar]
- Leino R. L., McCormick J. H. Morphological and morphometrical changes in chloride cells of the gills of Pimephales promelas after chronic exposure to acid water. Cell Tissue Res. 1984;236(1):121–128. doi: 10.1007/BF00216521. [DOI] [PubMed] [Google Scholar]
- Leivestad H., Muniz I. P. Fish kill at low pH in a Norwegian river. Nature. 1976 Feb 5;259(5542):391–392. doi: 10.1038/259391a0. [DOI] [PubMed] [Google Scholar]
- Lock R. A., van Overbeeke A. P. Effects of mercuric chloride and methylmercuric chloride on mucus secretion in rainbow trout, Salmo gairdneri Richardson. Comp Biochem Physiol C. 1981;69C(1):67–73. doi: 10.1016/0306-4492(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Miller T. G., Mackay W. C. Relationship of secreted mucus to copper and acid toxicity in rainbow trout. Bull Environ Contam Toxicol. 1982 Jan;28(1):68–74. doi: 10.1007/BF01608415. [DOI] [PubMed] [Google Scholar]
- Moseley R. H., Meier P. J., Aronson P. S., Boyer J. L. Na-H exchange in rat liver basolateral but not canalicular plasma membrane vesicles. Am J Physiol. 1986 Jan;250(1 Pt 1):G35–G43. doi: 10.1152/ajpgi.1986.250.1.G35. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Orellana S. A., Kimberg L. S., Field M., Halm D. R., Krasny E. J., Jr, Frizzell R. A. Na+-K+-Cl- co-transport in the intestine of a marine teleost. Nature. 1982 Nov 25;300(5890):351–353. doi: 10.1038/300351a0. [DOI] [PubMed] [Google Scholar]
- Pritchard J. B. Toxic substances and cell membrane function. Fed Proc. 1979 Jul;38(8):2220–2225. [PubMed] [Google Scholar]
- ROTHSTEIN A. Cell membrane as site of action of heavy metals. Fed Proc. 1959 Dec;18:1026–1038. [PubMed] [Google Scholar]
- Reuss L., Lewis S. A., Wills N. K., Helman S. I., Cox T. C., Boron W. F., Siebens A. W., Guggino W. B., Giebisch G., Schultz S. G. Ion transport processes in basolateral membranes of epithelia. Fed Proc. 1984 Jul;43(10):2488–2502. [PubMed] [Google Scholar]
- Sardet C., Pisam M., Maetz J. The surface epithelium of teleostean fish gills. Cellular and junctional adaptations of the chloride cell in relation to salt adaptation. J Cell Biol. 1979 Jan;80(1):96–117. doi: 10.1083/jcb.80.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P., Solomon R., Spokes K., Epstein F. Ouabain inhibition of gill Na-K-ATPase: relationship to active chloride transport. J Exp Zool. 1977 Mar;199(3):419–426. doi: 10.1002/jez.1401990316. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Greger R., Dunham P. B., Benjamin M. A., Frizzell R. A., Field M., Spring K. R., Ives H. E., Aronson P. S., Seifter J. Ion transport processes in apical membranes of epithelia. Fed Proc. 1984 Jul;43(10):2473–2487. [PubMed] [Google Scholar]
- Watson T. A., Beamish F. W. The effects of zinc on branchial adenosine triphosphatase enzymes in vitro from rainbow trout, Salmo Gairdneri. Comp Biochem Physiol C. 1981;68C(2):167–173. doi: 10.1016/0306-4492(81)90011-3. [DOI] [PubMed] [Google Scholar]
- Zuchelkowski E. M., Lantz R. C., Hinton D. E. Effects of acid-stress on epidermal mucous cells of the brown bullhead Ictalurus nebulosus (LeSeur): a morphometric study. Anat Rec. 1981 May;200(1):33–39. doi: 10.1002/ar.1092000104. [DOI] [PubMed] [Google Scholar]