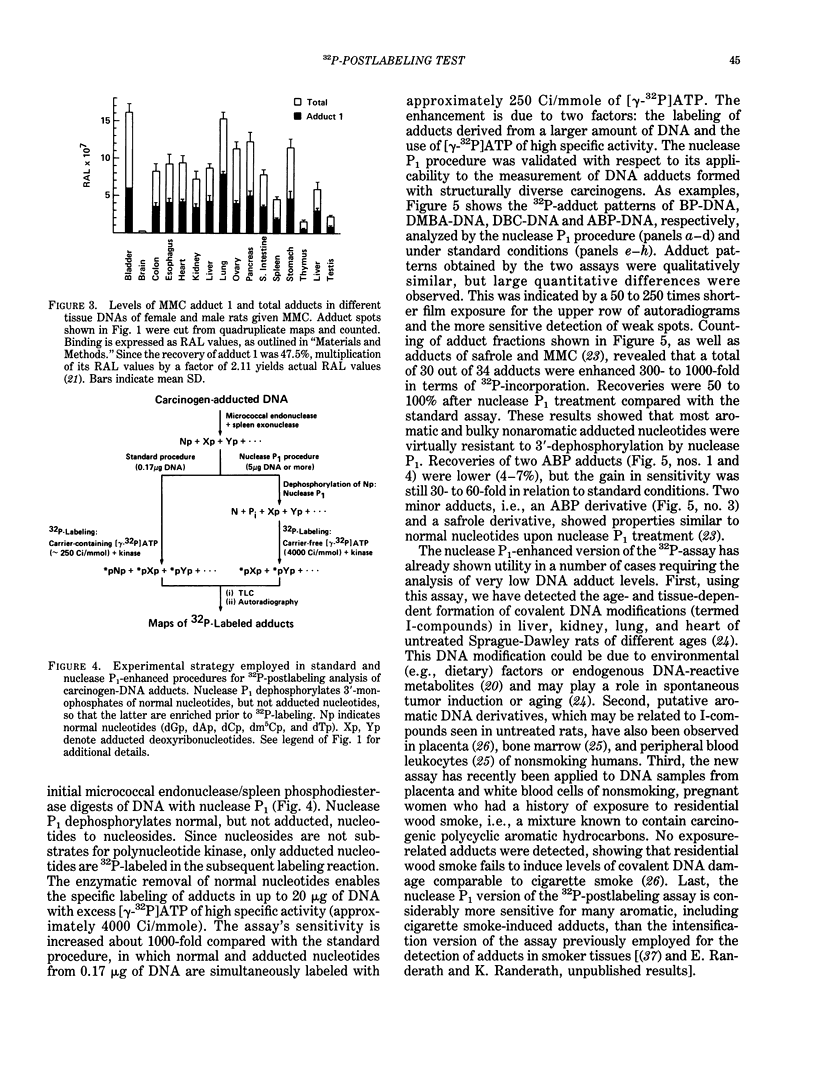
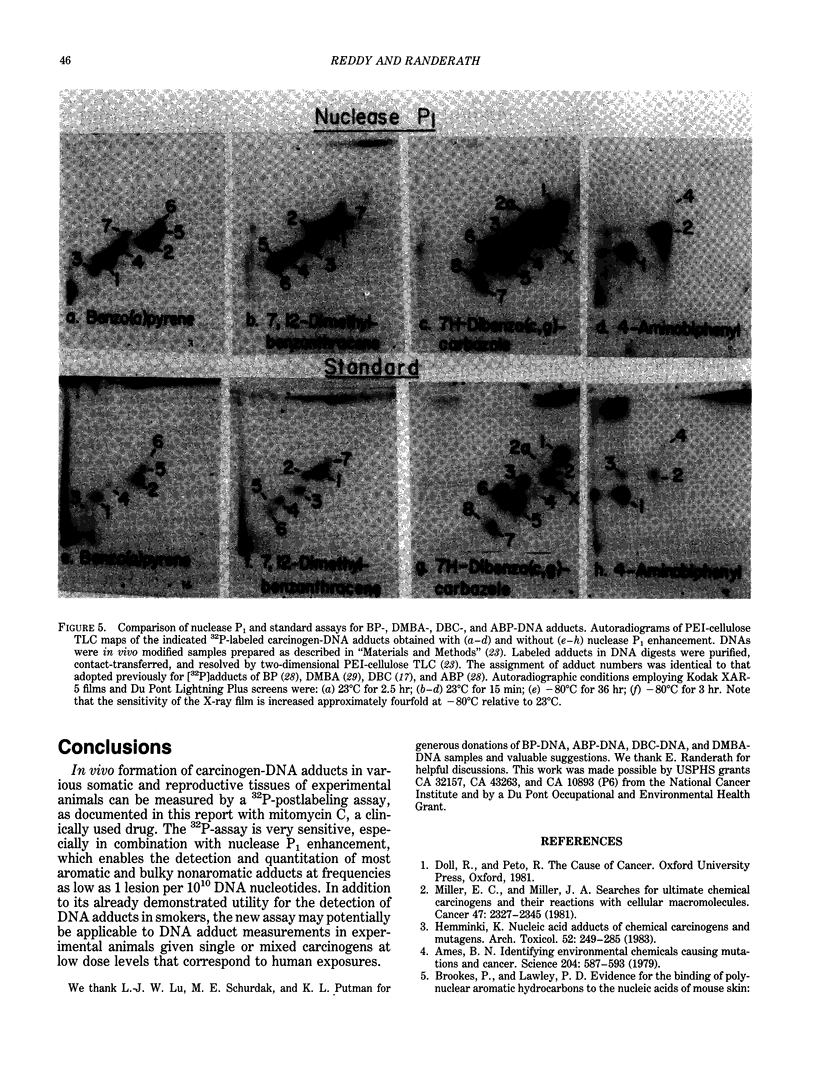
Abstract
Exceedingly sensitive assays are required for the detection of DNA adducts formed in humans exposed to low levels of environmental genotoxicants and therapeutic drugs. A 32P-postlabeling procedure for detection and quantitation of aromatic carcinogen-DNA lesions with a sensitivity limit of 1 adduct in 10(7) to 10(8) nucleotides has been described previously. In the standard procedure, DNA is enzymatically digested to 3'-phosphorylated normal and adducted mononucleotides, which are 32P-labeled at 5'-hydroxyl groups by T4 polynucleotide kinase-catalyzed [32P]phosphate transfer from [gamma-32P]ATP. 32P-labeled derivatives are resolved by TLC, detected by autoradiography, and quantitated by counting. This assay has been recently utilized for the determination and partial characterization of DNA adducts formed in somatic and reproductive tissues of rats given the clinically used anticancer drug, mitomycin C. The drug exhibits similar levels of covalent binding to DNA in most tissues. Further studies have revealed that adducted nucleotides are primarily guanine derivatives that are resistant to 3'-dephosphorylation by Penicillium citrinum nuclease P1. The latter observation has been utilized to enhance the 32P-assay's sensitivity to 1 adduct in 10(10) nucleotides for a 10-micrograms DNA sample by postincubation of DNA digests with nuclease P1 before 32P-labeling. The enzyme dephosphorylates the normal nucleotides but not most aromatic and bulky nonaromatic adducts, so that only the latter serve as substrates for the kinase-catalyzed labeling reaction. The new assay has also shown utility in the analysis of very low levels of age- and tissue-related DNA modifications, which might arise from dietary or endogenous compounds, in untreated rats and in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Identifying environmental chemicals causing mutations and cancer. Science. 1979 May 11;204(4393):587–593. doi: 10.1126/science.373122. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Identifying environmental chemicals causing mutations and cancer. Science. 1979 May 11;204(4393):587–593. doi: 10.1126/science.373122. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Doll D. C., Weiss R. B., Issell B. F. Mitomycin: ten years after approval for marketing. J Clin Oncol. 1985 Feb;3(2):276–286. doi: 10.1200/JCO.1985.3.2.276. [DOI] [PubMed] [Google Scholar]
- Everson R. B., Randerath E., Santella R. M., Cefalo R. C., Avitts T. A., Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 1986 Jan 3;231(4733):54–57. doi: 10.1126/science.3941892. [DOI] [PubMed] [Google Scholar]
- Fennell T. R., Juhl U., Miller E. C., Miller J. A. Identification and quantitation of hepatic DNA adducts formed in B6C3F1 mice from 1'-hydroxy-2',3'-dehydroestragole: comparison of the adducts detected with the 1'-3H-labelled carcinogen and by 32P-postlabelling. Carcinogenesis. 1986 Nov;7(11):1881–1887. doi: 10.1093/carcin/7.11.1881. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Nucleic acid adducts of chemical carcinogens and mutagens. Arch Toxicol. 1983 Apr;52(4):249–285. doi: 10.1007/BF00316495. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Nucleic acid adducts of chemical carcinogens and mutagens. Arch Toxicol. 1983 Apr;52(4):249–285. doi: 10.1007/BF00316495. [DOI] [PubMed] [Google Scholar]
- Liehr J. G., Avitts T. A., Randerath E., Randerath K. Estrogen-induced endogenous DNA adduction: possible mechanism of hormonal cancer. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5301–5305. doi: 10.1073/pnas.83.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. J., Disher R. M., Reddy M. V., Randerath K. 32P-postlabeling assay in mice of transplacental DNA damage induced by the environmental carcinogens safrole, 4-aminobiphenyl, and benzo(a)pyrene. Cancer Res. 1986 Jun;46(6):3046–3054. [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- PHILIPS F. S., SCHWARTZ H. S., STERNBERG S. S. Pharmacology of mitomycin C. I. Toxicity and pathologic effects. Cancer Res. 1960 Oct;20:1354–1361. [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Grover P. L. Aromatic DNA adducts in human bone marrow and peripheral blood leukocytes. Carcinogenesis. 1986 Dec;7(12):2071–2075. doi: 10.1093/carcin/7.12.2071. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Reddy M. V., Randerath K. 32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. II. Newborn male B6C3F1 mice. Carcinogenesis. 1984 Dec;5(12):1623–1628. doi: 10.1093/carcin/5.12.1623. [DOI] [PubMed] [Google Scholar]
- Poirier M. C. Antibodies to carcinogen-DNA adducts. J Natl Cancer Inst. 1981 Sep;67(3):515–519. [PubMed] [Google Scholar]
- Randerath E., Agrawal H. P., Weaver J. A., Bordelon C. B., Randerath K. 32P-postlabeling analysis of DNA adducts persisting for up to 42 weeks in the skin, epidermis and dermis of mice treated topically with 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1985 Aug;6(8):1117–1126. doi: 10.1093/carcin/6.8.1117. [DOI] [PubMed] [Google Scholar]
- Randerath K., Haglund R. E., Phillips D. H., Reddy M. V. 32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. I. Adult female CD-1 mice. Carcinogenesis. 1984 Dec;5(12):1613–1622. doi: 10.1093/carcin/5.12.1613. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Agrawal H. P., Gupta R. C., Schurdak M. E., Reddy M. V. Postlabeling methods for carcinogen-DNA adduct analysis. Environ Health Perspect. 1985 Oct;62:57–65. doi: 10.1289/ehp.856257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Ion-exchange thin-layer chromatography. XV. Preparation, properties and applications of paper-like PEI-cellulose sheets. J Chromatogr. 1966 Apr;22(1):110–117. doi: 10.1016/s0021-9673(01)97076-1. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Disher R. M. Age- and tissue-related DNA modifications in untreated rats: detection by 32P-postlabeling assay and possible significance for spontaneous tumor induction and aging. Carcinogenesis. 1986 Sep;7(9):1615–1617. doi: 10.1093/carcin/7.9.1615. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Gupta R. C., Randerath E., Randerath K. 32P-postlabeling test for covalent DNA binding of chemicals in vivo: application to a variety of aromatic carcinogens and methylating agents. Carcinogenesis. 1984 Feb;5(2):231–243. doi: 10.1093/carcin/5.2.231. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Gupta R. C., Randerath K. 32P-base analysis of DNA. Anal Biochem. 1981 Nov 1;117(2):271–279. doi: 10.1016/0003-2697(81)90722-3. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Irvin T. R., Randerath K. Formation and persistence of sterigmatocystin--DNA adducts in rat liver determined via 32P-postlabeling analysis. Mutat Res. 1985 Oct;152(1):85–96. [PubMed] [Google Scholar]
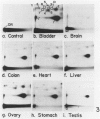
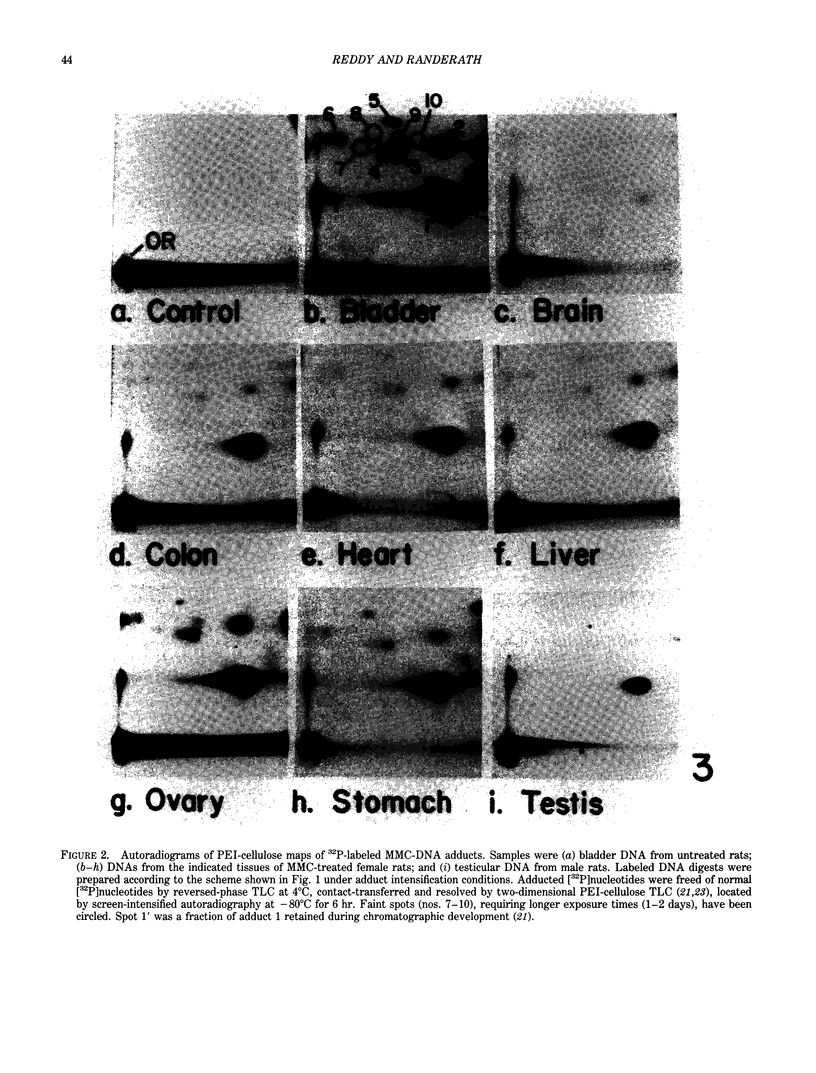
- Reddy M. V., Randerath K. 32P-analysis of DNA adducts in somatic and reproductive tissues of rats treated with the anticancer antibiotic, mitomycin C. Mutat Res. 1987 Jul;179(1):75–88. doi: 10.1016/0027-5107(87)90043-1. [DOI] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Schmähl D., Osswald H. Experimentelle Untersuchungen über carcinogene Wirkungen von Krebs-Chemotherapeutica und Immunosuppressiva. Arzneimittelforschung. 1970 Oct;20(10):1461–1467. [PubMed] [Google Scholar]
- Schurdak M. E., Stong D. B., Warshawsky D., Randerath K. 32P-postlabeling analysis of DNA adduction in mice by synthetic metabolites of the environmental carcinogen, 7H-dibenzo[c,g]carbazole: chromatographic evidence for 3-hydroxy-7H-dibenzo[c,g]carbazole being a proximate genotoxicant in liver but not skin. Carcinogenesis. 1987 Apr;8(4):591–597. doi: 10.1093/carcin/8.4.591. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Chowdary D., Lipman R., Shimotakahara S., Veiro D., Walker V., Verdine G. L. Reaction of DNA with chemically or enzymatically activated mitomycin C: isolation and structure of the major covalent adduct. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6702–6706. doi: 10.1073/pnas.83.18.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz M., Lipman R., Chowdary D., Pawlak J., Verdine G. L., Nakanishi K. Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science. 1987 Mar 6;235(4793):1204–1208. doi: 10.1126/science.3103215. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Bos J. L., Marshall C. J., Phillips D. H. Mutations activating human c-Ha-ras1 protooncogene (HRAS1) induced by chemical carcinogens and depurination. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1222–1226. doi: 10.1073/pnas.83.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]