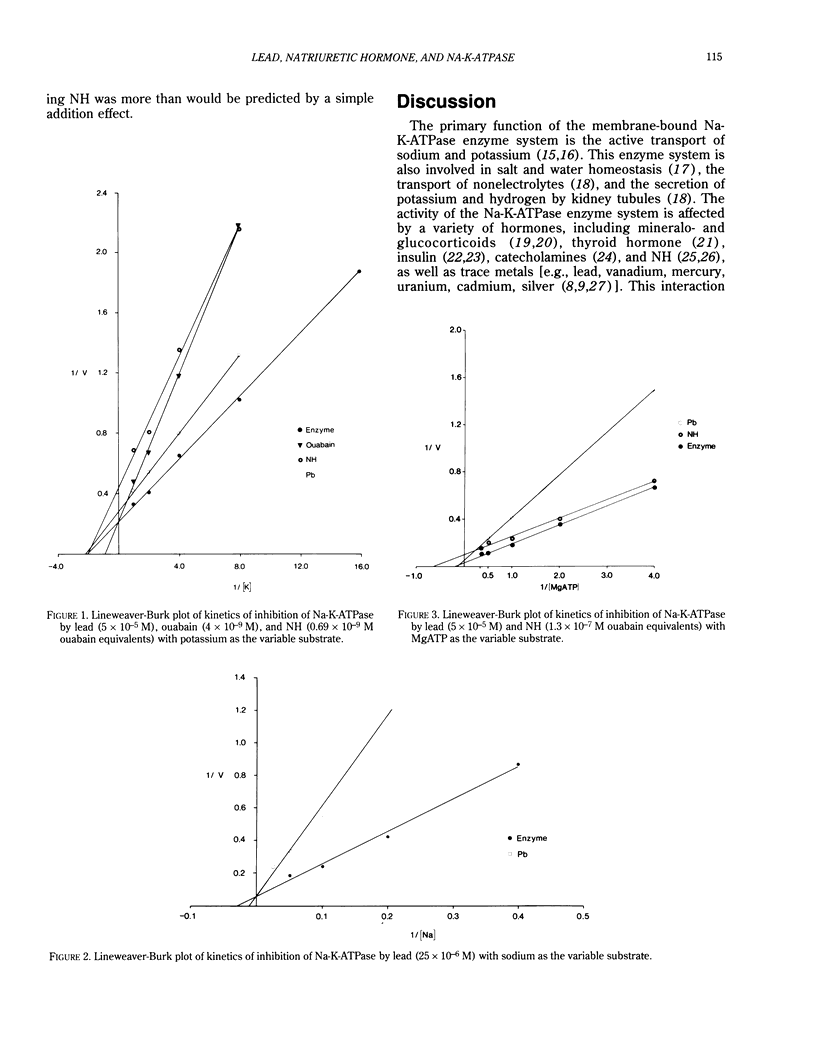
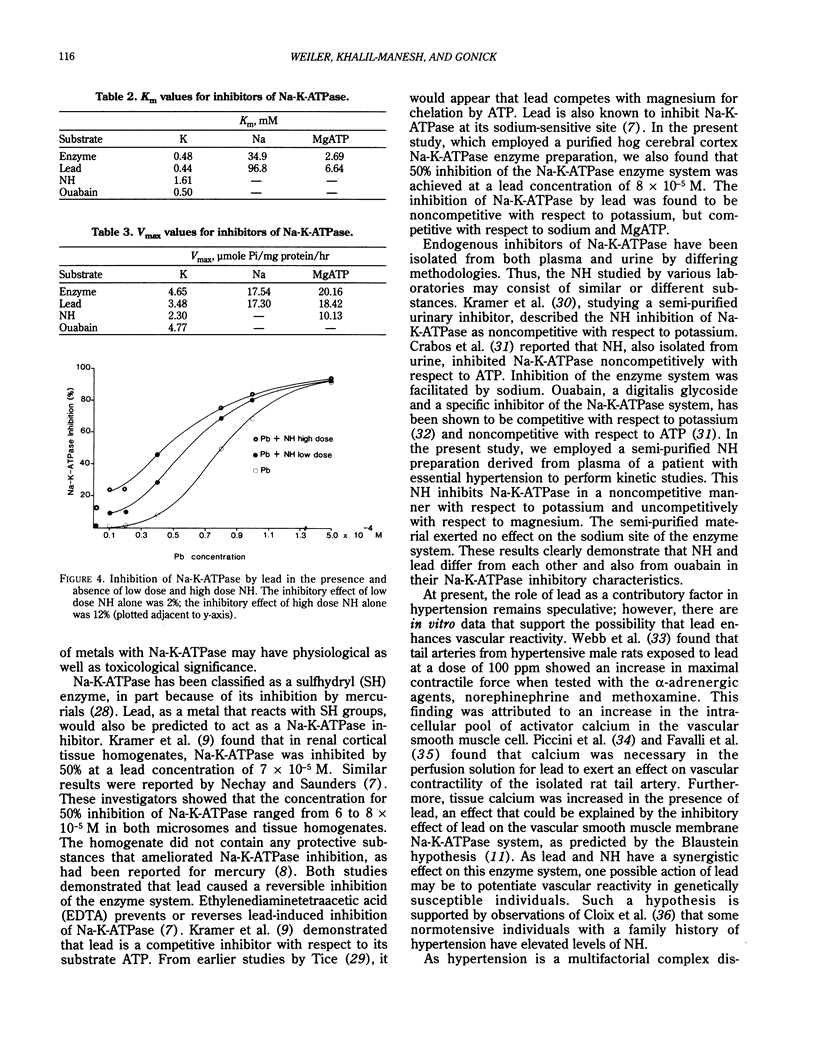
Abstract
Inhibition of vascular smooth muscle sodium-potassium-activated adenosine triphosphatase (Na-K-ATPase) has been postulated as a central mechanism in enhancing vascular contractility. In the present study, kinetics of inhibition of Na-K-ATPase by lead, ouabain, and natriuretic hormone (NH) was studied in a purified hog cerebral cortex enzyme preparation. Determination of I50 values for lead, ouabain, and NH revealed that NH is the most potent inhibitor of the enzyme system (0.8 x 10(-6) M ouabain equivalents). Kinetic analyses indicated that lead and NH exhibited different inhibitory mechanisms. The inhibition by lead was noncompetitive with respect to potassium and competitive with respect to sodium and MgATP. Natriuretic hormone was noncompetitive with respect to potassium, uncompetitive with respect to MgATP, and exhibited no inhibitory effect with respect to sodium. Synergism between lead and NH in the inhibition of Na-K-ATPase raises the possibility that lead may be a contributory factor in hypertension via this mechanism.
Full text
PDF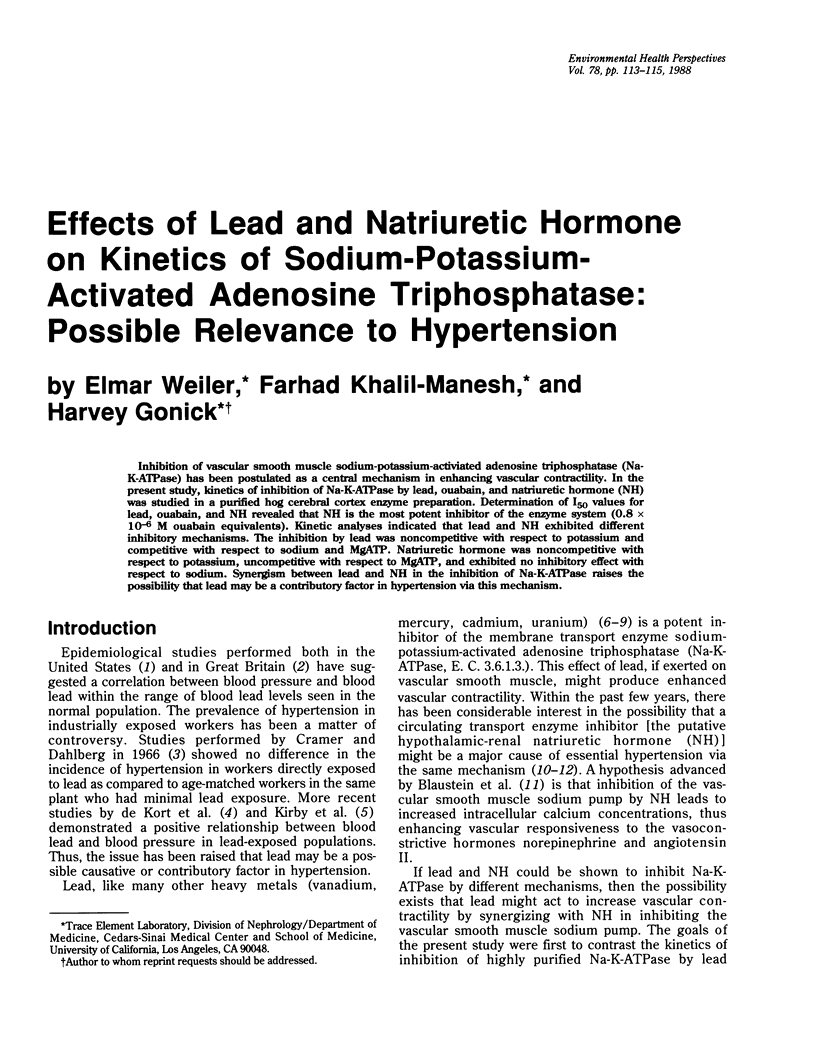


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P. Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977 May;232(5):C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- Buckalew V. M., Jr, Nelson D. B. Natriuretic and sodium transport inhibitory activity in plasma of volume-expanded dogs. Kidney Int. 1974 Jan;5(1):12–22. doi: 10.1038/ki.1974.2. [DOI] [PubMed] [Google Scholar]
- Cloix J. F., Devynck M. A., Elghozi J. L., Kamal L. A., Lacerda-Jacomini L. C., Meyer P., Pernollet M. G., Rosenfeld J. B., De Thé H. Plasma endogenous sodium pump inhibitor in essential hypertension. J Hypertens Suppl. 1983 Dec;1(2):11–14. [PubMed] [Google Scholar]
- Crabos M., Grichois M. L., Guicheney P., Wainer I. W., Cloix J. F. Further biochemical characterization of an Na+ pump inhibitor purified from human urine. Eur J Biochem. 1987 Jan 2;162(1):129–135. doi: 10.1111/j.1432-1033.1987.tb10552.x. [DOI] [PubMed] [Google Scholar]
- Cramér K., Dahlberg L. Incidence of hypertension among lead workers. A follow-up study based on regular control over 20 years. Br J Ind Med. 1966 Apr;23(2):101–104. doi: 10.1136/oem.23.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favalli L., Chiari M. C., Piccinini F., Rozza A. Experimental investigations on the contraction induced by lead in arterial smooth muscle. Acta Pharmacol Toxicol (Copenh) 1977;41 (Suppl 2):412–420. [PubMed] [Google Scholar]
- Gavryck W. A., Moore R. D., Thompson R. C. Effect of insulin upon membrane-bound (Na+ + K+)-ATPase extracted from frog skeletal muscle. J Physiol. 1975 Oct;252(1):43–58. doi: 10.1113/jphysiol.1975.sp011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERA F. C., WHITTEMBURY G., PLANCHART A. Effect of insulin on short-circuit current across isolated frog skin in the presence of calcium and magnesium. Biochim Biophys Acta. 1963 Jan 15;66:170–172. doi: 10.1016/0006-3002(63)91182-x. [DOI] [PubMed] [Google Scholar]
- Haddy F. J., Pamnani M. B., Clough D. L. Humoral factors and the sodium-potassium pump in volume expanded hypertension. Life Sci. 1979 Jun 4;24(23):2105–2117. doi: 10.1016/0024-3205(79)90108-5. [DOI] [PubMed] [Google Scholar]
- Haddy F. J. Sodium-potassium pump in low-renin hypertension. Ann Intern Med. 1983 May;98(5 Pt 2):781–784. doi: 10.7326/0003-4819-98-5-781. [DOI] [PubMed] [Google Scholar]
- Hart W. M., Jr, Titus E. O. Sulfhydryl groups of sodium-potassium transport adenosine triphosphatase. Protection by physiological ligands and exposure by phosphorylation. J Biol Chem. 1973 Jul 10;248(13):4674–4681. [PubMed] [Google Scholar]
- Jørgensen P. L. Sodium and potassium ion pump in kidney tubules. Physiol Rev. 1980 Jul;60(3):864–917. doi: 10.1152/physrev.1980.60.3.864. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Lindheimer M. D. Renal sodium- and potassium-activated adenosine triphosphatase and sodium reabsorption in the hypothyroid rat. J Clin Invest. 1973 Apr;52(4):796–804. doi: 10.1172/JCI107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby H., Gyntelberg F. Blood pressure and other cardiovascular risk factors of long-term exposure to lead. Scand J Work Environ Health. 1985 Feb;11(1):15–19. doi: 10.5271/sjweh.2259. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Baecker A., Weiler E., Liddiard C. Studies on ouabain-like endogenous natriuretic factors in human urine. Inhibition of Na-K-ATPase and 3H-ouabain binding. Klin Wochenschr. 1986 Aug 15;64(16):760–766. doi: 10.1007/BF01734344. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Gonick H. C., Lu E. In vitro inhibition of Na-K-ATPase by trace metals: relation to renal and cardiovascular damage. Nephron. 1986;44(4):329–336. doi: 10.1159/000184015. [DOI] [PubMed] [Google Scholar]
- Mujais S. K., Chekal M. A., Jones W. J., Hayslett J. P., Katz A. I. Regulation of renal Na-K-ATPase in the rat. Role of the natural mineralo- and glucocorticoid hormones. J Clin Invest. 1984 Jan;73(1):13–19. doi: 10.1172/JCI111183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechay B. R. Biochemical basis of diuretic action. J Clin Pharmacol. 1977 Oct;17(10 Pt 2):626–641. [PubMed] [Google Scholar]
- Nechay B. R., Nelson J. A., Contreras R. R., Sarles H. E., Remmers A. R., Jr, beathard G. A., Jr, fish J. C., Lindley J. D., Brady J. M., Lerman M. J. Ouabain-sensitive adenosine triphosphatase from human kidneys. J Pharmacol Exp Ther. 1975 Feb;192(2):303–309. [PubMed] [Google Scholar]
- Nechay B. R., Saunders J. P. Inhibition by vanadium of sodium and potassium dependent adenosinetriphosphatase derived from animal and human tissues. J Environ Pathol Toxicol. 1978 Nov-Dec;2(2):247–262. [PubMed] [Google Scholar]
- Nechay B. R., Saunders J. P. Inhibition of adenosine triphosphatase in vitro by silver nitrate and silver sulfadiazine. J Environ Pathol Toxicol Oncol. 1984 Jul;5(4-5):119–126. [PubMed] [Google Scholar]
- Nechay B. R., Saunders J. P. Inhibitory characteristics of cadmium, lead, and mercury in human sodium and potassium dependent adenosinetriphosphatase preparations. J Environ Pathol Toxicol. 1978 Nov-Dec;2(2):283–290. [PubMed] [Google Scholar]
- Nechay B. R., Saunders J. P. Inhibitory characteristics of lead chloride in sodium- and potassium- dependent adenosinetriphosphatase preparations derived from kidney, brain, and heart of several species. J Toxicol Environ Health. 1978 Jan;4(1):147–159. doi: 10.1080/15287397809529652. [DOI] [PubMed] [Google Scholar]
- Piccinini F., Favalli L., Chiari M. C. Experimental investigations on the contraction induced by lead in arterial smooth muscle. Toxicology. 1977 Aug;8(1):43–51. doi: 10.1016/0300-483x(77)90022-1. [DOI] [PubMed] [Google Scholar]
- Pirkle J. L., Schwartz J., Landis J. R., Harlan W. R. The relationship between blood lead levels and blood pressure and its cardiovascular risk implications. Am J Epidemiol. 1985 Feb;121(2):246–258. doi: 10.1093/oxfordjournals.aje.a113995. [DOI] [PubMed] [Google Scholar]
- Pocock S. J., Shaper A. G., Ashby D., Delves T., Whitehead T. P. Blood lead concentration, blood pressure, and renal function. Br Med J (Clin Res Ed) 1984 Oct 6;289(6449):872–874. doi: 10.1136/bmj.289.6449.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogus E., Price T., Zierler K. L. Sodium plus potassium-activated, ouabain-inhibited adenosine triphosphatase from a fraction of rat skeletal muscle, and lack of insulin effect on it. J Gen Physiol. 1969 Aug;54(2):188–202. doi: 10.1085/jgp.54.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Tice L. W. Lead-adenosine triphosphate complexes in adenosine triphosphatase histochemistry. J Histochem Cytochem. 1969 Feb;17(2):85–94. doi: 10.1177/17.2.85. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Winquist R. J., Victery W., Vander A. J. In vivo and in vitro effects of lead on vascular reactivity in rats. Am J Physiol. 1981 Aug;241(2):H211–H216. doi: 10.1152/ajpheart.1981.241.2.H211. [DOI] [PubMed] [Google Scholar]
- Westenfelder C., Arevalo G. J., Baranowski R. L., Kurtzman N. A., Katz A. I. Relationship between mineralocorticoids and renal Na+-K+-ATPase: sodium reabsorption. Am J Physiol. 1977 Dec;233(6):F593–F599. doi: 10.1152/ajprenal.1977.233.6.F593. [DOI] [PubMed] [Google Scholar]
- de Kort W. L., Verschoor M. A., Wibowo A. A., van Hemmen J. J. Occupational exposure to lead and blood pressure: a study in 105 workers. Am J Ind Med. 1987;11(2):145–156. doi: 10.1002/ajim.4700110204. [DOI] [PubMed] [Google Scholar]
- de Wardener H. E., MacGregor G. A. The relation of a circulating sodium transport inhibitor (the natriuretic hormone?) to hypertension. Medicine (Baltimore) 1983 Sep;62(5):310–326. doi: 10.1097/00005792-198309000-00004. [DOI] [PubMed] [Google Scholar]


