Figure 2.
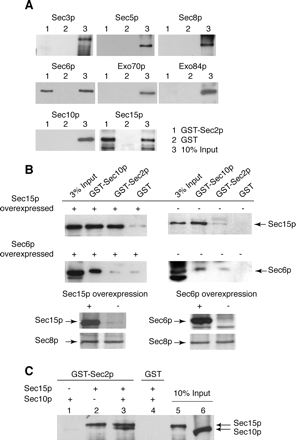
The Sec15p subunit of the exocyst complex interacts with GST-Sec2p. (A) Full-length GST-Sec2p was purified from E. coli strain BL21. As a control, GST protein was also purified from E. coli. GST-Sec2p (lanes 1) and GST (lanes 2) immobilized on glutathione-Sepharose beads (20 μl of 50% slurry, the amount of the fusion protein on beads is 2.5 μg) were incubated with the different exocyst subunits (0.5 μg) individually purified from E. coli. The binding reaction was performed in a buffer containing 1× PBS, 1 mg/ml BSA, and 0.25% Triton X-100 for 1 h at room temperature. Lanes 3 represents 10% of the input of each individually purified exocyst subunit. His6-exocyst subunits were detected with monoclonal αHis (Novagen). (B) Co-overexpression of GST-Sec2p and Sec15p in yeast results in increased coprecipitation of Sec15p with GST-Sec2p. Top left, GST-Sec2p was co-overexpressed with Sec15p in yeast (NY2525) and isolated using glutathione-Sepharose beads. As a control GST-Sec10p (a known Sec15p interacting protein; positive control) and GST (negative control) were also co-overexpressed with Sec15p (NY2526 and NY2527, respectively). Top right, similar GST pull downs, except that the amount of Sec15p is at native level (NY2528, NY2529, and NY2530). In the Sec15p-overexpressing yeast strains, the amount of Sec15p is 20-fold higher than native amount. Middle, this experiment was analogous to the top experiment, except that in this case, Sec6p was co-overexpressed with GST-Sec2p (NY2550), GST-Sec10p (NY2551), and GST (NY 2552). The exposure times for left and right panels are different. Bottom, amount of Sec15p and Sec6p in overproducing and nonoverproducing yeast lysates. Sec8p is shown as a loading control. In all these experiments, cells were grown overnight in YP + 2% glycerol and induced by adding 2% galactose for 8 h. (C) Binding sites for Sec2p and Sec10p on Sec15p are nonoverlapping. GST-Sec2p immobilized on glutathione beads was incubated with Sec10p (lane 1), Sec15p (lane 2), or both (lane3). Control binding to GST is shown in lane 4. All proteins were expressed and purified from bacteria. The binding reaction was performed as in A. Lanes 5 and 6 represent 10% of input of Sec15p and Sec10p, respectively.