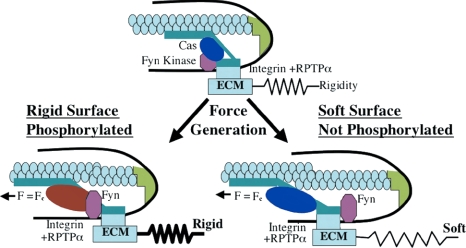
Figure 7.
Model of rigidity response though RPTPα-mediated Fyn activation. Schematic drawing depicts how RPTPα-mediated activation of Fyn upon cell adhesion to FN-coated substrate regulates rigidity response. Cells attach to rigid or soft FN-coated substrate through αvβ3 integrins, which causes formation of the integrin–cytoskeleton complex at the leading edge. This complex is connected to acto-myosin gel via actin-binding proteins in the complex. Inactive Fyn (pink) is bound to membrane due to palmitoylation. Force is exerted on the leading edge complex in response to matrix rigidity. At low forces, RPTPα is activated by a conformation change in the αvβ3 integrin, and it subsequently activates Fyn by dephosphorylating it. Simultaneously, rearward movement of actin begins driven by myosin motors. The leading edge complex moves along with the actin network, but at a slower rate because of the slip bond between integrins and cytoskeleton. On rigid substrates force is developed rapidly; therefore, it reaches the critical force (Fc) required for Fyn or Cas activation while all components are in close proximity. On soft substrates, the displacement needed to reach the activation force threshold is greater; therefore, inactive Cas (blue) moves away from Fyn before force-dependent activation can cause phosphorylation. On rigid substrates, phosphorylation of Cas (red) is followed by accumulation of focal contact proteins and reinforcement of the integrin–cytoskeleton bond. This stabilizes leading edge and accelerates cell spreading. On soft substrates, unphosphorylated Cas fails to play its role in reinforcement, which results in impaired spreading.