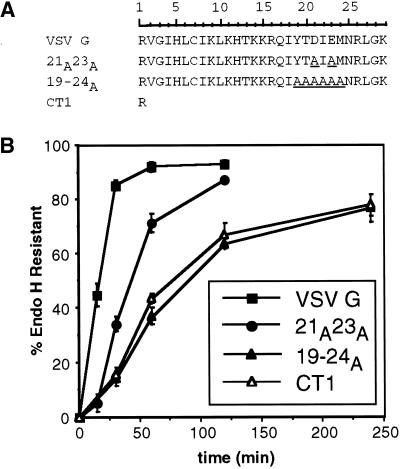
Figure 1.
Efficient intracellular transport of VSV G requires six residues in the cytoplasmic tail. (A) Amino acid sequence (single-letter code) of the entire cytoplasmic domain of wild-type and mutant VSV G proteins. The scale above the primary sequence of the VSV G tail indicates the residue number corresponding to each amino acid of the cytoplasmic domain. Residue 1 follows the last residue of the transmembrane domain, whereas residue 29 corresponds to the C-terminal amino acid of the tail. Substituted residues in mutant tails are underlined. (B) Kinetics of acquisition of endo H resistance of VSV G, 21A23A, 19–24A, and CT1. BHK-21 cells expressing VSV G or mutant VSV G proteins were pulse labeled for 5 min and chased for the times indicated. Immunoprecipitated proteins were subjected to endo H treatment as described in MATERIALS AND METHODS and separated by SDS-PAGE. The amount of endo H-resistant protein was quantitated by phosphorimaging. Each time point represents the mean of a minimum of three experiments ± SD.