Abstract
Glycinebetaine (hereafter referred to as betaine) is a compatible solute that accumulates in certain plants and microorganisms in response to various types of stress. We demonstrated previously that when the cyanobacterium Synechococcus sp. PCC 7942 (hereafter Synechococcus) is transformed with the codA gene for choline oxidase, it can synthesize betaine from exogenously supplied choline, exhibiting enhanced tolerance to salt and cold stress. In this study, we examined the effects of salt stress and betaine synthesis on the photoinhibition of photosystem II (PSII). Salt stress due to 220 mm NaCl enhanced photoinhibition of PSII and betaine protected PSII against photoinhibition under these conditions. However, neither salt stress nor betaine synthesis affected photodamage to PSII. By contrast, salt stress inhibited repair of photodamaged PSII and betaine reversed this inhibitory effect of salt stress. Pulse-chase-labeling experiments revealed that salt stress inhibited degradation of D1 protein in photodamaged PSII and de novo synthesis of D1. By contrast, betaine protected the machinery required for degradation and synthesis of D1 under salt stress. Neither salt stress nor betaine affected levels of psbA transcripts. These observations suggest that betaine counteracts the inhibitory effects of salt stress, with resultant accelerated repair of photodamaged PSII.
Living organisms are frequently exposed to various kinds of environmental stress in their natural habitats and they have developed mechanisms that allow them to withstand such stress. One such mechanism involves the accumulation of compatible solutes, which are defined as water-soluble organic compounds of low molecular mass that are nontoxic at high concentrations (Chen and Murata, 2002).
Glycinebetaine (hereafter referred to as betaine) is a compatible solute; it is an amphoteric compound and extremely soluble in water. The molecular features of betaine allow it to interact with both the hydrophobic and the hydrophilic domains of macromolecules. Results of studies in vitro indicate that betaine stabilizes the structure and activity of enzymes and maintains the integrity of membranes against the damaging effects of excessive salt, cold, heat, and freezing (Gorham, 1995). Betaine is found in a wide variety of prokaryotes, eukaryotic microorganisms, higher plants, and animals (Wilken et al., 1970; Galinski and Trüper, 1982; Yancey et al., 1982; Mohammad et al., 1983; Hanson et al., 1985). In a comprehensive investigation of the actions of betaine in vivo, we transformed plants, namely, Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), and tomato (Lycopersicon esculentum), with the codA gene for choline oxidase, which catalyzes the synthesis of betaine from choline. The resultant transgenic plants synthesized and accumulated betaine and exhibited, in addition, enhanced tolerance to various environmental stresses, such as high salt, high and low temperatures, and freezing (Hayashi et al., 1997, 1998; Alia et al., 1998a, 1998b, 1999; Sakamoto and Murata, 2001a, 2001b ; Chen and Murata, 2002; Sulpice et al., 2003; Park et al., 2004).
We also transformed the cyanobacterium Synechococcus sp. PCC 7942 (hereafter Synechococcus) with the codA gene. The line of transformed Synechococcus cells, which we designated PAMCOD, synthesized betaine in vivo from exogenously supplied choline and accumulated betaine at levels of 60 to 80 mm (Deshnium et al., 1995). PAMCOD cells that are grown in the presence of 1 mm choline can continue to grow in the presence of 400 mm NaCl, whereas wild-type cells, which do not synthesize betaine from choline, cannot grow at all at this concentration of NaCl. PAMCOD cells exhibit similarly enhanced tolerance to low temperatures when supplied with exogenous choline. This enhanced tolerance to low temperature was ascribed to acceleration of the repair of photodamaged PSII and not to protection of PSII against photodamage (Deshnium et al., 1997).
In this study, we attempted to elucidate the mechanism whereby synthesis of betaine protects PAMCOD cells from salt stress. We found that betaine protected cyanobacterial cells against the synergistic actions of salt stress and strong light stress by accelerating repair of PSII. This acceleration was related to enhancement of the degradation of D1 protein in photodamaged PSII and de novo synthesis of D1.
RESULTS
Betaine Protected Synechococcus against Photoinhibition of PSII under Salt Stress
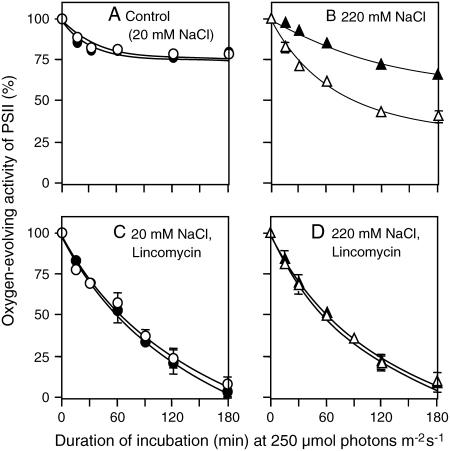
In previous studies, we demonstrated that PAMCOD cells, namely, Synechococcus cells that had been transformed with the codA gene for choline oxidase, were able to synthesize betaine and accumulate this compatible solute in the cytosol at levels of 60 to 80 mm when choline was supplied exogenously as the substrate for betaine synthesis (Deshnium et al., 1995). Therefore, we examined the effects of strong light and 220 mm NaCl on PAMCOD cells grown in the absence of choline (control cells) and on PAMCOD cells grown in medium supplemented with 1.0 mm choline chloride, the substrate for betaine synthesis (choline-supplemented cells). Figure 1A shows that, when PAMCOD cells were exposed to strong light at 250 μmol photons m−2 s−1 under control conditions (20 mm NaCl), PSII activity decreased only slightly at the early stage of photoinhibition, but remained high subsequently. Synthesis of betaine, as a result of the availability of choline, did not affect photoinhibition of PSII under normal conditions.
Figure 1.
Betaine protected PSII against photoinhibition under salt stress in the PAMCOD line of Synechococcus cells. PAMCOD cells at 3 μg of chlorophyll mL−1 were exposed to strong white light at 250 μmol photons m−2 s−1 from an incandescent lamp in the presence of 20 mm NaCl (control conditions) or 220 mm NaCl (salt stress conditions) and in the presence or absence of 250 μg mL−1 lincomycin. To establish salt stress at 220 mm NaCl, a small portion of 5 m NaCl was added to cell cultures (i.e. 4% [v/v]) 3 min before exposure to strong light. PSII activity was measured by monitoring the oxygen-evolving activity in the presence of 1,4-benzoquinone as the artificial acceptor of electrons. The activity corresponding to 100% was 860 ± 34 μmol O2 (mg chlorophyll)−1 h−1. White (○, ▵) and black (•, ▴) symbols represent control cells that had been unable to synthesize betaine and choline-supplemented cells that had accumulated betaine, respectively. A, Control (20 mm NaCl). B, 220 mm NaCl. C, 20 mm NaCl + 250 μg mL−1 lincomycin. D, 220 mm NaCl + 250 μg mL−1 lincomycin. Each point and error bar represents the average and sd, respectively, of results from three independent experiments.
Figure 1B shows that under salt stress due to the presence of 220 mm NaCl, photoinhibition of PSII was significantly enhanced. In particular, PSII activity decreased to 40% of the initial level during a 180-min incubation period. The apparent acceleration of photoinhibition under salt stress was consistent with our previous observations in Synechocystis sp. PCC 6803 (Allakhverdiev et al., 2002). In choline-supplemented cells, PSII activity fell to about 70% of the initial level during incubation in light at 250 μmol photons m−2 s−1 for 180 min. This result indicated that betaine, synthesized in vivo from exogenous choline, protected PSII against photoinhibition under salt stress.
Salt Stress Had No Effect on Photodamage to PSII But Inhibited Repair of PSII
Because the extent of photoinhibition depends on the balance between photodamage to PSII and repair of such damage, we postulated that it might be possible to explain the stimulation and repression of photoinhibition by salt stress and betaine synthesis, respectively, in terms of regulation of photodamage and/or repair. To identify the steps that were affected by the actions of salt stress and betaine, we examined photodamage and repair separately.
Photodamage to PSII can be examined separately from repair of PSII by use of lincomycin, an inhibitor of protein synthesis. Figure 1C shows that, in the presence of lincomycin, PSII activity declined rapidly during incubation in light at 250 μmol photons m−2 s−1 and that synthesis of betaine did not affect photodamage to PSII. Figure 1D shows that salt stress and synthesis of betaine did not affect the decrease in PSII activity that was due to photodamage. These results indicate that salt stress did not affect photodamage to PSII. Moreover, they were consistent with results obtained in Synechocystis (Allakhverdiev et al., 2002; Allakhverdiev and Murata, 2004) and, in addition, they showed that betaine did not protect PSII from photodamage.
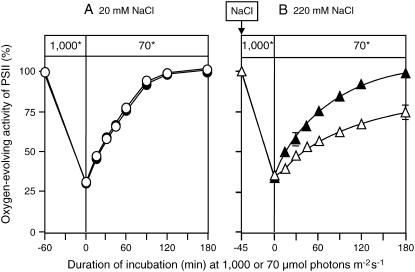
The results in Figure 1 suggested that the protective effect of betaine on PSII activity might have been due to enhancement of the repair of PSII after photodamage. Therefore, we examined the effects of betaine synthesis on repair of PSII after photodamage. We exposed PAMCOD cells to strong light at 1,000 μmol photons m−2 s−1 for 60 min in the presence of 20 mm NaCl or for 45 min in the presence of 220 mm NaCl to reduce PSII activity to 30% of the initial level in each case, and then we transferred the cultures to weak light at 70 μmol photons m−2 s−1 for recovery (Fig. 2). In the absence of salt stress, PSII activity returned to the original level during incubation for 120 min and betaine did not affect this repair process (Fig. 2A). By contrast, salt stress significantly inhibited repair of PSII. During incubation in weak light for 180 min, PSII activity recovered to only approximately 70% of the initial level (Fig. 2B). However, the presence of betaine in choline-supplemented cells enhanced repair of PSII considerably, and complete recovery of PSII activity was observed after incubation for 180 min (Fig. 2B). Thus, the presence of betaine in the cytosol clearly reversed the inhibitory effects of salt stress on repair of photodamaged PSII.
Figure 2.
Betaine accelerated repair of photodamaged PSII under salt stress. PAMCOD cells were exposed to strong light at 1,000 μmol photons m−2 s−1 to decrease PSII activity to 30% of the original level and then transferred to weak light at 70 μmol photons m−2 s−1 to allow repair of photodamaged PSII. White (○, ▵) and black (•, ▴) symbols represent control cells that were unable to synthesize betaine and choline-supplemented cells that had accumulated betaine, respectively. Other experimental conditions were the same as described in the legend to Figure 1. A, Control (20 mm NaCl). B, Salt stress conditions (220 mm NaCl). The asterisk (*) indicates that the intensity of light is given in μmol photons m−2 s−1. Each point and error bar represents the average and sd, respectively, of results from three independent experiments.
Betaine Accelerated Degradation of D1 Protein under Salt Stress
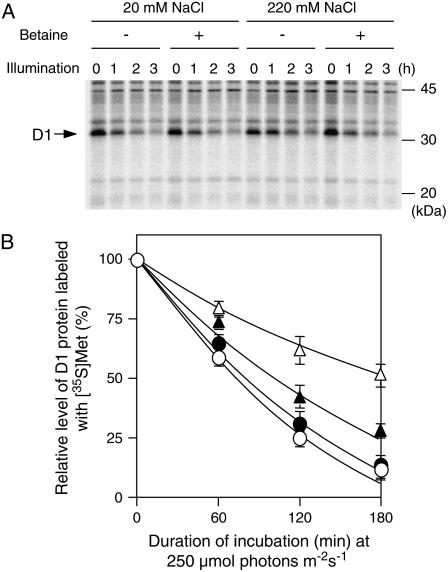
Repair of photodamaged PSII involves several steps and degradation of D1 protein is the first of these steps. We examined the effects of salt stress and betaine on degradation of D1 in photodamaged PSII by pulse-chase analysis with radioactively labeled Met (Fig. 3). Figure 3A shows that, during incubation in light at 250 μmol photons m−2 s−1, the level of pulse-labeled D1 decreased and the labeled protein almost disappeared during incubation for 180 min under control conditions. The level of labeled D1 in control cells decreased under salt stress more slowly than under control conditions, suggesting that salt stress due to 220 mm NaCl delayed degradation of D1 protein. By contrast, the decrease in the level of labeled D1 in choline-supplemented cells under salt stress was similar to that observed under control conditions.
Figure 3.
Betaine accelerated degradation of D1 protein in photodamaged PSII under salt stress (pulse-chase experiment). PAMCOD cells were pulse labeled with [35S]Met and radioactivity was pulse chased during exposure to light at 250 μmol photons m−2 s−1, as described in “Materials and Methods.” Thylakoid membranes were isolated from cells that had been harvested at designated times and the proteins in the thylakoid membranes were solubilized and separated by SDS-PAGE as described in “Materials and Methods.” A, Autoradiogram. B, Time course of changes in levels of D1 protein that had been labeled with [35S]Met during incubation in light at 250 μmol photons m−2 s−1. ○, Control cells (20 mm NaCl); •, choline-supplemented cells (20 mm NaCl); ▵, control cells (220 mm NaCl); ▴, choline-supplemented cells (220 mm NaCl). Each point and error bar represents the average and sd, respectively, of results from three independent experiments.
The time course of the degradation of D1 protein (Fig. 3B) revealed that the rate of degradation of this protein was unaffected by the presence of intracellular betaine under control conditions. Under salt stress, there was an obvious decrease in the rate of degradation of D1 protein in control cells. However, this effect of 220 mm NaCl was barely evident in choline-supplemented cells. These observations suggest that salt stress inhibited degradation of D1 protein and that betaine counteracted the inhibitory effects of NaCl on degradation of this protein.
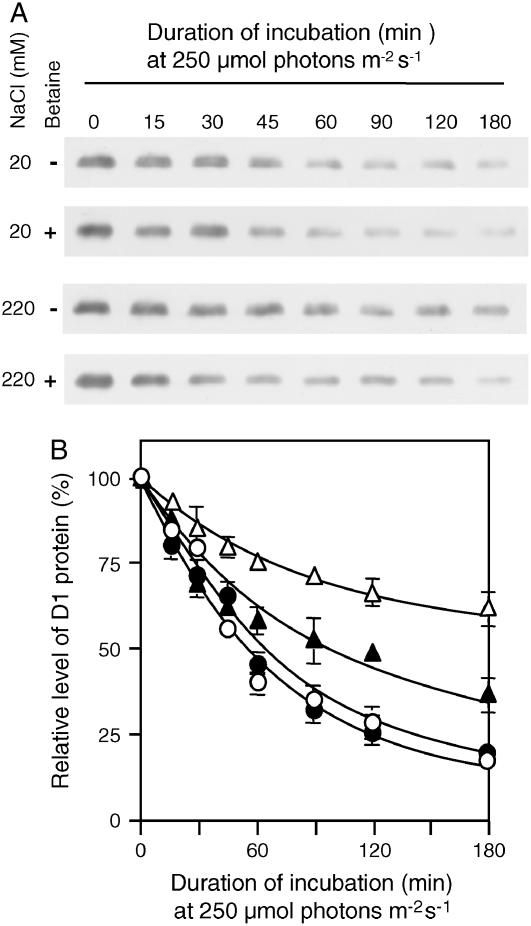
We also analyzed changes in the level of D1 protein by western blotting. It has been suggested that degradation and de novo synthesis of D1 protein might be coupled (Komenda and Barber, 1995). Thus, the inhibition by salt stress of the degradation of D1 might have been reflected by the inhibitory effect of NaCl on the de novo synthesis of D1. To evaluate this possibility, we estimated the rate of degradation of D1 protein by western-blot analysis under conditions where degradation was separated from de novo synthesis by inclusion of lincomycin. Figure 4 shows that, in the presence of lincomycin, the level of D1 decreased to approximately 20% of the original value during incubation in strong light for 180 min under control conditions (20 mm NaCl). The presence of betaine had no effect on the rate of degradation of D1 protein. Salt stress due to 220 mm NaCl significantly retarded degradation of D1 in control cells. Moreover, even in the presence of lincomycin, the rate of degradation of D1 under salt stress was increased in choline-supplemented cells. These observations confirmed the opposing effects of salt stress and betaine on degradation of D1 protein. They also suggested that the effects of salt stress and betaine on degradation of D1 are independent of the de novo synthesis of D1.
Figure 4.
Betaine accelerated degradation of D1 protein in photodamaged PSII under salt stress. PAMCOD cells were exposed to light at 250 μmol photons m−2 s−1 in the presence of 250 μg mL−1 lincomycin. Thylakoid membranes were isolated from cells that had been harvested at designated times. Proteins in thylakoid membranes were solubilized and separated by SDS-PAGE as described in “Materials and Methods.” After blotting onto a nitrocellulose membrane, the proteins were allowed to react with D1-specific antibodies. A, Western blot of D1 protein. B, Time course of changes in levels of D1 protein during incubation in light at 250 μmol photons m−2 s−1. ○, Control cells (20 mm NaCl); •, choline-supplemented cells (20 mm NaCl); ▵, control cells (220 mm NaCl); ▴, choline-supplemented cells (220 mm NaCl). Each point and error bar represents the average and sd, respectively, of results from three independent experiments.
Betaine Accelerated de Novo Synthesis of D1 Protein under Salt Stress
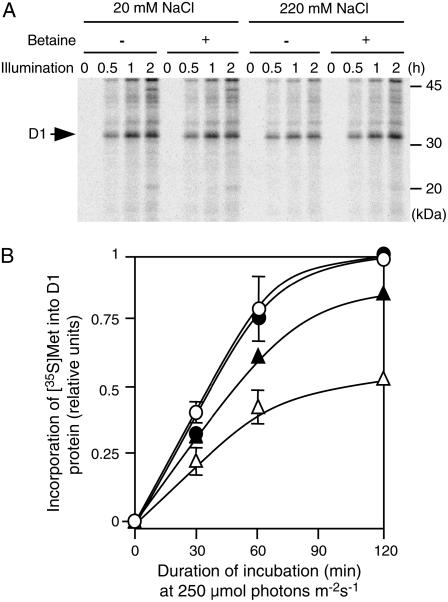
We examined the effects of salt stress and betaine on de novo synthesis of D1 protein by pulse labeling with [35S]Met. Figure 5A shows changes in the labeling patterns of thylakoid-membrane proteins during photoinhibition in the presence of 20 and 220 mm NaCl. Under control conditions (20 mm NaCl), de novo synthesis of thylakoid-membrane proteins was unaffected by the presence of exogenous choline. By contrast, salt stress (220 mm NaCl) clearly decreased the levels of radioactively labeled proteins. However, in choline-supplemented cells, radioactively labeled proteins in thylakoid membranes remained at levels similar to those observed under control conditions. The time course of changes in the level of radioactively labeled D1 (Fig. 5B) showed that betaine did not affect de novo synthesis of D1 in light under nonstress conditions (20 mm NaCl). By contrast, under salt stress (220 mm NaCl), synthesis of D1 was significantly inhibited. However, the presence of choline improved the efficiency of synthesis of D1. These results suggest that salt stress inhibits de novo synthesis of D1 and that betaine reverses the inhibitory effects of salt stress on synthesis of D1 protein.
Figure 5.
Betaine accelerated de novo synthesis of D1 protein during photoinhibition under salt stress. PAMCOD cells were labeled with [35S]Met during incubation in light at 250 μmol photons m−2 s−1 as described in “Materials and Methods.” Thylakoid membranes were isolated from cells that had been harvested at designated times and proteins in thylakoid membranes were solubilized and separated by SDS-PAGE as described in “Materials and Methods.” A, Autoradiogram. B, Time course of changes in levels of D1 protein that had been labeled with [35S]Met during incubation in light at 250 μmol photons m−2 s−1. ○, Control cells (20 mm NaCl); •, choline-supplemented cells (20 mm NaCl); ▵, control cells (220 mm NaCl); ▴, choline-supplemented cells (220 mm NaCl). Each point and error bar represents the average and sd, respectively, of results from three independent experiments.
We examined the effects of salt stress and betaine on the level of psbA transcripts by northern blotting. The genome of Synechococcus includes three psbA genes, designated psbAI, psbAII, and psbAIII. Whereas only the psbAI gene is expressed under normal conditions in light at 70 μmol photons m−2 s−1, expression of the psbAII and psbAIII genes is induced and that of psbAI mRNAs is repressed upon exposure of Synechococcus cells to strong light at 250 μmol photons m−2 s−1 (Golden et al., 1986; Kulkarni et al., 1992). The results of northern blotting indicated that such changes in the expression of the psbAI, psbAII, and psbAIII genes occurred under all sets of conditions examined, suggesting that neither salt stress nor betaine synthesis affected the transcription of psbA genes (data not shown). Therefore, we can conclude that the inhibition and enhancement of synthesis of the D1 protein, as shown in Figure 5, were not caused by any effects of salt stress and betaine synthesis on transcription of the psbA genes.
DISCUSSION
Hypothetical Scheme for the Effects of Salt Stress and Betaine on Photoinhibition
In this study, we demonstrated that salt stress due to 220 mm NaCl repressed repair of photodamaged PSII by inhibiting degradation and synthesis of D1 protein. We also demonstrated that the presence of exogenously supplied choline reversed the inhibitory effect of 220 mm NaCl on repair of PSII by accelerating degradation and de novo synthesis of D1 protein. It is reasonable to assume that this effect of choline is related to the synthesis and accumulation of betaine in PAMCOD cells, namely, transformed Synechococcus cells that express choline oxidase, synthesize betaine from choline, and accumulate betaine at levels of 60 to 80 mm (Deshnium et al., 1995). Moreover, the protective effect of betaine appeared when repair of PSII was repressed by salt stress.
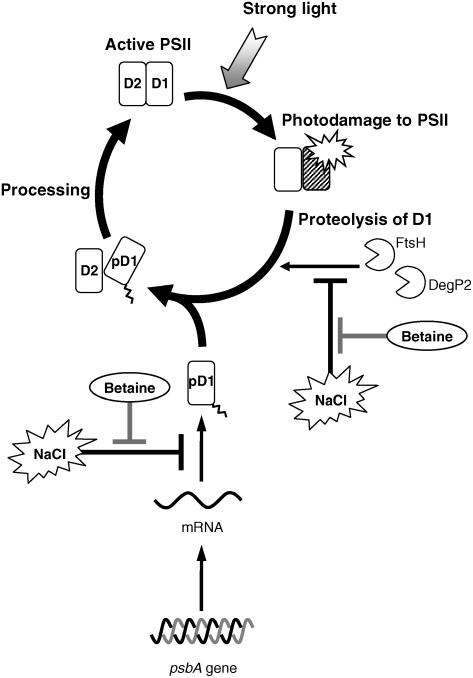
Figure 6 shows a hypothetical scheme for the actions of NaCl and betaine. Photodamage to D1 protein, which can be isolated from the repair process by lincomycin, was unaffected by the presence of NaCl and betaine. Only degradation of D1 protein and translation of psbA mRNAs are influenced by NaCl and betaine.
Figure 6.
Hypothetical scheme for the effects of salt stress and betaine on the photodamage-repair cycle of PSII during photoinhibition. In this scheme, salt stress inhibits both degradation of D1 protein and de novo synthesis of the pre-D1 (pD1). Betaine reverses these inhibitory effects of salt stress, with resultant enhancement of the repair of PSII under salt stress conditions.
Possible Mechanisms for the Effects of Salt Stress on Degradation and de Novo Synthesis of D1 Protein
Degradation of D1 protein is one of the earliest in the repair of photodamaged PSII. Genetic and biochemical approaches have demonstrated that two proteases are involved in this degradation, namely, FtsH (Lindahl et al., 2000; Silva et al., 2003) and DegP2 (Haußühl et al., 2001; Silva et al., 2002). The two proteases probably act in concert to degrade D1. This study demonstrated that salt stress due to 220 mm NaCl inhibits degradation of D1 in Synechococcus (Figs. 3 and 4). The molecular mechanism responsible for such inhibition is unclear. However, it seems possible that salt stress might inhibit interaction between these proteases and D1 or that salt stress might inactivate these proteases. It has been reported that FtsH participates in the formation of large protein complexes of approximately 500 kD in Synechocystis sp. PCC 6803 (Silva et al., 2003). A homolog of FtsH in Arabidopsis, VAR2, which is involved in degradation of D1 protein (Bailey et al., 2002), forms a protein complex of 400 to 450 kD with VAR1, which is another homolog of FtsH (Sakamoto et al., 2003). Salt stress might dissociate the constituents of such large complexes. DegP2 is bound to the stromal side of thylakoid membranes and is released from isolated thylakoid membranes by treatment with NaCl (Haußühl et al., 2001). Salt stress might dissociate DegP2 from thylakoid membranes in Synechococcus with resultant inhibition of degradation of D1 protein.
De novo synthesis of D1 protein is an important event in the repair of PSII (Aro et al., 1993). Synthesis of D1 requires transcription of psbA genes, translation of psbA transcripts to yield the precursor to D1 protein (pre-D1), incorporation of pre-D1 into the PSII complex, and processing of pre-D1 to generate mature D1 protein. In a previous study of Synechocystis sp. PCC 6803, we demonstrated that salt stress due to 500 mm NaCl inhibits translation of psbA transcripts, but does not affect transcription of psbA genes (Allakhverdiev et al., 2002). In this study, we found that salt stress due to 220 mm NaCl inhibited translation of psbA transcripts, but did not affect transcription of psbA genes in Synechococcus. The difference with respect to the concentration of NaCl that is effective in the two cyanobacteria might be related to the fact that Synechococcus is more sensitive to salt stress than Synechocystis sp. PCC 6803.
Translation of psbA transcripts is a strongly regulated process and many regulatory factors are probably involved in both initiation and elongation. In yeast (Saccharomyces cervisiae), initiation of translation involves a variety of regulatory factors, such as eukaryotic initiation factor (eIF) 3 and eIF4E (Pyronnet and Sonenberg, 2001), and initiation is a target of inhibition by salt stress (Uesono and Toh-E, 2002). The genome of Synechococcus includes genes for the translation initiation factors of prokaryotic type, namely, IF1 and IF2 (GenBank accession no. NC_006576; available at cyano.genome.jp). Therefore, a similar inhibitory action of salt stress might occur in Synechococcus cells. Salt stress might inhibit the interaction of nucleotides with the regulatory protein complex and/or the formation of functional protein complexes.
Peptide elongation is also controlled at several steps (Kim et al., 1991; Tyystjärvi et al., 2001) and, in Synechococcus, one such step is the targeting of the psbA mRNA-ribosome complex to the thylakoid membrane (Tyystjärvi et al., 2001). Under strong light and low-temperature stress, psbAII and psbAIII mRNAs are translated, whereas psbAI mRNA is not, when these transcripts are associated with membrane-bound ribosomes (Tyystjärvi et al., 2004). Thus, elongation during translation probably involves a number of regulatory factors and the elongation step could also be a site of inhibition by salt stress. It remains to be determined whether it is the initiation step or the elongation step in the translation of psbA transcripts that is inhibited by salt stress in Synechococcus. Identification of factors that are most sensitive to salt stress requires further investigation of the mechanism that regulates synthesis of D1 protein.
Betaine Counteracts the Effects of Salt Stress on Degradation and de Novo Synthesis of D1 Protein
Betaine counteracted the effects of NaCl on degradation of D1 protein (Fig. 4). As shown in Figures 3 and 5, we observed the effects of betaine and NaCl not only on the turnover of D1 protein, but also on that of some other proteins that bind to thylakoid membranes; these proteins might have contributed, in part, to the repair of the PSII complex. Therefore, it seems likely that both NaCl and betaine might affect the proteolytic and translational machinery. Opposing effects of betaine and NaCl have also been observed in the synthesis of soluble proteins in nonphotosynthetic bacteria, such as Staphylococcus aureus (Vijaranakul et al., 1997) and Enterococcus faecalis (Pichereau et al., 1999).
NaCl impedes the intermolecular association of protein subunits and betaine might compete with NaCl and enhance such an association. The integrity and activity of the translational machinery might be reduced by salt stress and enhanced by betaine. There is ample evidence that betaine stabilizes the conformation of proteins and, in particular, the quaternary structure of complex proteins. For example, betaine protects the oxygen-evolving complex of PSII from the NaCl- and heat-induced dissociation of extrinsic proteins (Papageorgiou and Murata, 1995).
Two hypotheses have been proposed for the mechanism whereby betaine stabilizes macromolecular structures. One hypothesis involves the formation of a hydration shell (layers of bound water) around the surface of proteins (Arakawa and Timasheff, 1983) and the other involves the direct stabilization of proteins by betaine (Schobert, 1977). In Synechococcus, betaine might be able to stabilize proteases and the translational machinery by one or both mechanisms even under salt stress.
MATERIALS AND METHODS
Microorganism and Culture Conditions
Synechococcus sp. PCC 7942 cells were transformed with the codA gene for choline oxidase (X84895) as described previously (Deshnium et al., 1995) and the resultant mutant cells were designated PAMCOD cells. They were grown at 34°C in BG11 medium that contained 20 mm NaCl in the presence of 1.0 mm choline chloride (choline-supplemented cells) or in its absence (control cells) under illumination from incandescent lamps at 70 μmol photons m−2 s−1 with aeration by air that contained 1% CO2. Cells were grown to a concentration that corresponded to approximately 5 μg chlorophyll mL−1. An aliquot was withdrawn and diluted to a chlorophyll concentration of 3 μg mL−1 with BG11 medium that contained 1.0 mm choline chloride or no choline. After incubation for 60 min under the same conditions as above, cells were used for experiments.
Measurement of PSII Activity
We measured PSII activity at 34°C in BG11 medium in terms of the photosynthetic evolution of oxygen by monitoring the concentration of oxygen with a Clark-type oxygen electrode (Hansatech Instruments) in the presence of 1 mm 1,4-benzoquinone as the electron acceptor (Ono and Murata, 1981). Actinic light at 1,500 μmol photons m−2 s−1 was provided by an incandescent lamp equipped with a heat-absorbing filter (HA-50; Hoya Glass) and a red optical filter (R-60; Toshiba).
Preparation of Thylakoid Membranes
Four-milliliter aliquots of suspensions of cells at 3 μg chlorophyll mL−1 were withdrawn from cultures at designated times during incubation in strong light and centrifuged in Eppendorf tubes at 15,000g for 10 min. Each cell pellet was suspended in 180 μL of 50 mm Tris-HCl (pH 8.0) and 330 mg of glass beads (106 μm; Sigma-Aldrich) were added. The cells were disrupted by vortexing for 5 min with 30-s intervals between each minute of vortexing. The homogenate was transferred to a new Eppendorf tube with 1 mL of 50 mm Tris-HCl (pH 8.0) and then subjected to centrifugation at 15,000g for 10 min. The resultant pellet of thylakoid membranes was resuspended in 60 μL of 50 mm Tris-HCl (pH 8.0) that contained 25% (w/v) glycerol.
Western-Blot Analysis
Proteins in thylakoid membranes were solubilized by incubation at 65°C for 5 min in the presence of 2% SDS and 0.1 m dithiothreitol (2.5 μL of a mixture of 10% SDS and 0.5 m dithiothreitol were added to 10 μL of a suspension of thylakoid membranes that corresponded to 1 μg of chlorophyll). The solubilized proteins were separated by SDS-PAGE on a 15% polyacrylamide gel that contained 6 m urea. Separated polypeptides were blotted electrophoretically onto a nitrocellulose membrane and probed with polyclonal antibodies raised in rabbits against the full-length D1 protein from spinach (Spinacia oleracea; Vermass et al., 1988). Signals were visualized with an enhanced chemiluminescence reagent (ECL; Amersham Biosciences). Antibodies raised against D1 protein were kindly provided by Dr. M. Ikeuchi (University of Tokyo).
Pulse-Labeling and Pulse-Chase Experiments
Synechococcus cells that had been grown to a concentration that corresponded to approximately 5 μg chlorophyll mL−1 were collected by centrifugation at 7,500g for 10 min at 30°C and then resuspended in fresh BG11 medium. Cells resuspended in BG11 medium at 3 μg chlorophyll mL−1 were incubated at 34°C for 1 h in light at 70 μmol photons m−2 s−1 with aeration by air that contained 1% CO2.
In pulse-labeling experiments, cells were subsequently exposed to light at 250 μmol photon m−2 s−1 with stirring by a magnetic stirrer in the presence of 5 nm [35S]Met (5 μCi mL−1; Amersham Biosciences). In pulse-chase experiments, after incubation with aeration as above, cells were incubated at 34°C for 10 min in light at 70 μmol photons m−2 s−1 with stirring by a magnetic stirrer. After a 10-min incubation, cells were supplemented with 5 nm [35S]Met and incubated for another hour under the same conditions as above. Immediately prior to exposure to light at 250 μmol photons m−2 s−1, nonradioactive Met was added to a final concentration of 5 mm.
Pulse labeling and pulse chase were terminated by addition of lincomycin at 250 μg mL−1 and immediate cooling of samples on ice. Thylakoid membranes were isolated as described above and the membranes equivalent to 10 ng of chlorophyll were fractionated by SDS-PAGE on a 15% polyacrylamide gel that contained 6 m urea. Labeled proteins were detected with a Bio-imaging analyzer (BAS2000; Fuji Photo Film). Levels of labeled D1 protein were estimated densitometrically.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number X84895.
Acknowledgments
We thank Dr. M. Ikeuchi of the University of Tokyo for providing antibodies against the full-length D1 protein.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Norio Murata (murata@nibb.ac.jp).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.076976.
References
- Alia, Hayashi H, Chen TH, Murata N (1998. a) Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ 21: 232–239 [Google Scholar]
- Alia, Hayashi H, Sakamoto A, Murata N (1998. b) Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J 16: 155–161 [DOI] [PubMed] [Google Scholar]
- Alia, Kondo Y, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen TH, Murata N (1999) Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol 40: 279–288 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803.Biochim Biophys Acta 1657: 23–32 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Nishiyama Y, Miyairi S, Yamamoto H, Inagaki N, Kanesaki Y, Murata N (2002) Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol 130: 1443–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa T, Timasheff SN (1983) Preferential interactions of proteins with solvent components in aqueous amino acid solutions. Arch Biochem Biophys 224: 169–177 [DOI] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C, Mann NH (2002) A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the photosystem II repair cycle in vivo. J Biol Chem 277: 2006–2011 [DOI] [PubMed] [Google Scholar]
- Chen TH, Murata N (2002) Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol 5: 250–257 [DOI] [PubMed] [Google Scholar]
- Deshnium P, Gombos Z, Nishiyama Y, Murata N (1997) The action in vivo of glycine betaine in enhancement of tolerance of Synechococcus sp. strain PCC 7942 to low temperature. J Bacteriol 179: 339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshnium P, Los DA, Hayashi H, Mustardy L, Murata N (1995) Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress. Plant Mol Biol 29: 897–907 [DOI] [PubMed] [Google Scholar]
- Galinski EA, Trüper HG (1982) Betaine, a compatible solute in the extremely halophilic phototrophic bacterium Ectothiorhodospira halochloris. FEMS Microbiol Lett 13: 357–360 [Google Scholar]
- Golden SS, Brusslan J, Haselkorn R (1986) Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J 5: 2789–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham J (1995) Betaines in higher plants: biosynthesis and role in stress metabolism. In RM Wallsgrove, ed, Amino Acids and Their Derivatives in Higher Plants. Cambridge University Press, Cambridge, pp 171–203
- Hanson AD, May AM, Grumet R, Bode J, Jamieson GC, Rhodes D (1985) Betaine synthesis in chenopods: localization in chloroplasts. Proc Natl Acad Sci USA 82: 3678–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haußühl K, Andersson B, Adamska I (2001) A chloroplast DegP2 protease performs the primary cleavage of the photodamaged D1 protein in plant photosystem II. EMBO J 20: 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Alia, Mustardy L, Deshnium P, Ida M, Murata N (1997) Transformation of Arabidopsis thaliana with the codA gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J 12: 133–142 [DOI] [PubMed] [Google Scholar]
- Hayashi H, Alia, Sakamoto A, Nonaka H, Chen THH, Murata N (1998) Enhanced germination under high-salt conditions of seeds of transgenic Arabidopsis with a bacterial gene (codA) for choline oxidase. J Plant Res 111: 357–362 [Google Scholar]
- Kim J, Klein PG, Mullet JE (1991) Ribosomes pause at specific sites during synthesis of membrane-bound chloroplast reaction center protein D1. J Biol Chem 266: 14931–14938 [PubMed] [Google Scholar]
- Komenda J, Barber J (1995) Comparison of psbO and psbH deletion mutants of Synechocystis PCC 6803 indicates that degradation of D1 protein is regulated by the QB site and dependent on protein synthesis. Biochemistry 34: 9625–9631 [DOI] [PubMed] [Google Scholar]
- Kulkarni RD, Schaefer MR, Golden SS (1992) Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol 174: 3775–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Spetea C, Hundal T, Oppenheim AB, Adam Z, Andersson B (2000) The thylakoid FtsH protease plays a role in the light-induced turnover of the photosystem II D1 protein. Plant Cell 12: 419–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad FAA, Reed RH, Stewart WDP (1983) The halophilic cyanobacterium Synechocystis DUN52 and its osmotic responses. FEMS Microbiol Lett 16: 287–290 [Google Scholar]
- Ono T, Murata N (1981) Chilling susceptibility of the blue-green alga Anacystis nidulans: effect of growth temperature. Plant Physiol 67: 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou GC, Murata N (1995) The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem-II complex. Photosynth Res 44: 243–252 [DOI] [PubMed] [Google Scholar]
- Park EJ, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen TH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40: 474–487 [DOI] [PubMed] [Google Scholar]
- Pichereau V, Bourot S, Flahaut S, Blanco C, Auffray Y, Bernard T (1999) The osmoprotectant glycine betaine inhibits salt-induced cross-tolerance towards lethal treatment in Enterococcus faecalis. Microbiology 145: 427–435 [DOI] [PubMed] [Google Scholar]
- Pyronnet S, Sonenberg N (2001) Cell-cycle-dependent translational control. Curr Opin Genet Dev 11: 13–18 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Murata N (2001. a) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25: 163–171 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Murata N (2001. b) The use of bacterial choline oxidase, a glycinebetaine-synthesizing enzyme, to create stress-resistant transgenic plants. Plant Physiol 125: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W, Zaltsman A, Adam Z, Takahashi Y (2003) Coordinated regulation and complex formation of YELLOW VARIEGATRED1 and YELLOW VARIEGATRED2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell 15: 2843–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobert B (1977) Is there an osmotic regulatory mechanism in algae and higher plants? J Theor Biol 68: 17–26 [DOI] [PubMed] [Google Scholar]
- Silva P, Choi YJ, Hassan HA, Nixon PJ (2002) Involvement of the HtrA family of proteases in the protection of the cyanobacterium Synechocystis PCC 6803 from light stress and in the repair of photosystem II. Philos Trans R Soc Lond B Biol Sci 357: 1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P, Thompson E, Bailey S, Kruse O, Mullineaux CW, Robinson C, Mann NH, Nixon PJ (2003) FtsH is involved in the early stages of repair of photosystem II in Synechocystis sp. PCC 6803. Plant Cell 15: 2152–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Tsukaya H, Nonaka H, Mustardy L, Chen TH, Murata N (2003) Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J 36: 165–176 [DOI] [PubMed] [Google Scholar]
- Tyystjärvi T, Herranen M, Aro EM (2001) Regulation of translation elongation in cyanobacteria: membrane targeting of the ribosome nascent-chain complexes controls the synthesis of D1 protein. Mol Microbiol 40: 476–484 [DOI] [PubMed] [Google Scholar]
- Tyystjärvi T, Sirpiö S, Aro EM (2004) Post-transcriptional regulation of the psbA gene family in the cyanobacterium Synechococcus sp. PCC 7942. FEBS Lett 576: 211–215 [DOI] [PubMed] [Google Scholar]
- Uesono Y, Toh-E A (2002) Transient inhibition of translation initiation by osmotic stress. J Biol Chem 277: 13848–13855 [DOI] [PubMed] [Google Scholar]
- Vermass WFJ, Ikeuchi M, Inoue Y (1988) Protein composition of the photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC6803. Photosynth Res 17: 97–113 [DOI] [PubMed] [Google Scholar]
- Vijaranakul U, Nadakavukaren MJ, Bayles DO, Wilkinson BJ, Jayaswal RK (1997) Characterization of an NaCl-sensitive Staphylococcus aureus mutant and rescue of the NaCl-sensitive phenotype by glycine betaine but not by other compatible solutes. Appl Environ Microbiol 63: 1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken DR, McMacken MR, Rodrquez A (1970) Choline and betaine aldehyde oxidation by rat liver mitochondria. Biochim Biophys Acta 216: 305–317 [DOI] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222 [DOI] [PubMed] [Google Scholar]