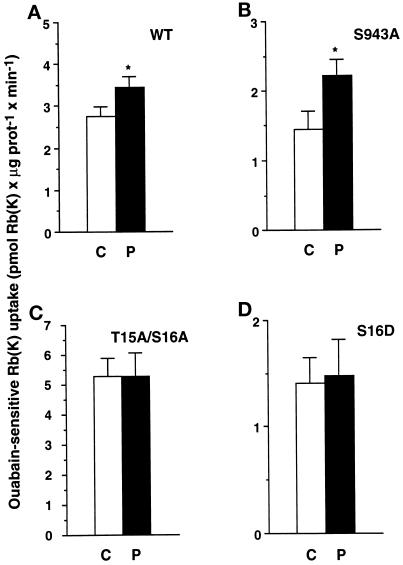
Figure 5.
In COS-7 cells incubated at 18°C, the PDBu-induced stimulation of the transport activity of the Na,K-ATPase is abolished by mutation of the α1 subunit at Ser-16. Exogenous Na,K-ATPase-mediated 86Rb uptake was measured in the presence of 2.5 × 10−6 M ouabain under initial rates of influx in COS-7 cells stably expressing the wild-type (A, WT) Bufo α1 subunit, the S943A (PKA site) mutant (B), the T15A/S16A mutant (C), or the S16D mutant mimicking constitutive phosphorylation (D). Cells were incubated for 30 min at 18°C in the absence (C, open bars) or in the presence of 10−7 M PDBu (P, filled bars). Results are expressed as picomoles of Rb (K) × microgram of protein−1 × minute−1 and are means ± SE from 7–20 independent experiments (*, p < 0.05).