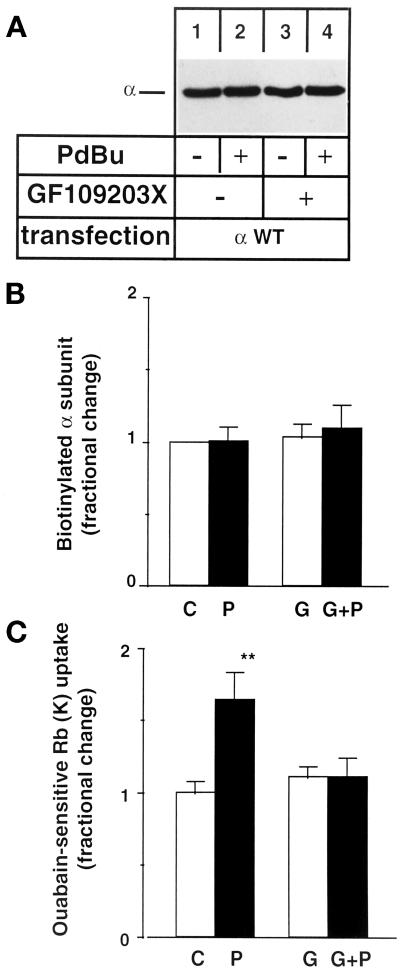
Figure 6.
Incubation at 18°C prevents the PDBu-induced down-regulation of cell surface Na,K-ATPase. COS-7 cells expressing wild-type Bufo α1 subunit were preincubated for 30 min at 18°C in the absence or in the presence of 10−7 M PDBu (P), 5 × 10−7 M GF109203X (G), or PDBu and GF109203X (G+P) before biotinylation of cell surface proteins (A and B) or measurement of exogenous Na,K-ATPase-mediated 86Rb uptake (C). After streptavidin precipitation, the cell surface–expressed Bufo α1 subunit was detected by immunoblot using a specific anti-Bufo α1 subunit antibody. (A) A representative immunoblot is shown (n = 4). (B) Quantitation of the relative amounts of Na,K-ATPase α1 subunit detected by immunoblotting. Results are expressed as fractional change (with respect to control value) and are means ± SE from four independent experiments. (C) Ouabain-sensitive 86Rb uptake. Results are expressed as fractional change (with respect to control value) and are means ± SE from 11 independent experiments (**, p < 0.01).