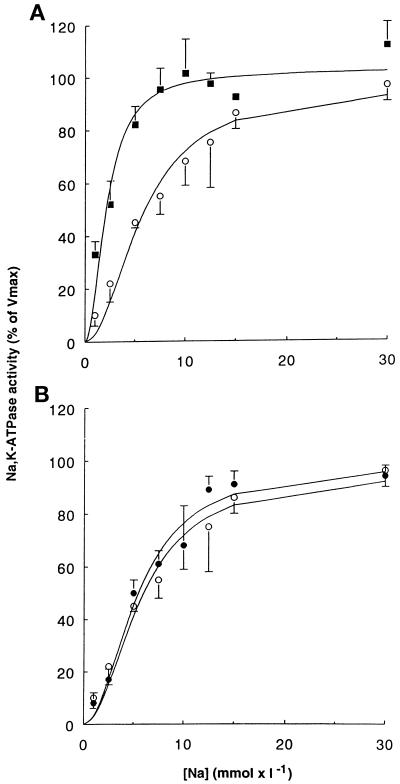
Figure 7.
A mutant mimicking phosphorylation of the α1 subunit at Ser-16 (S16D) increases the apparent Na affinity of Na,K-ATPase. The Na activation of the hydrolytic activity of the hybrid Na,K pumps was measured in permeabilized membranes isolated from stably transfected COS-7 cells. Results are expressed as the percentage of maximal activity and are means ± SE from four to seven independent experiments. (A) Na activation curves of Na,K-ATPase from cells expressing the wild-type Bufo α1 subunit (open circles) or the S16D α-mutant (closed squares). (B) Na activation curves of Na,K-ATPase from cells expressing the wild-type Bufo α1 subunit (open circles) or the T15A/S16A α-mutant (closed circles). The activity of exogenous Na,K-ATPase measured in the presence of 30 mM Na was 586 ± 9 (n = 6), 314 ± 47 (n = 4), and 631 ± 85 (n = 5) pmol of ATP × h−1 × μg of protein−1 for the wild-type, the S16D mutant, and the T15A/S16A mutant Na,K-pumps, respectively.