Figure 3.
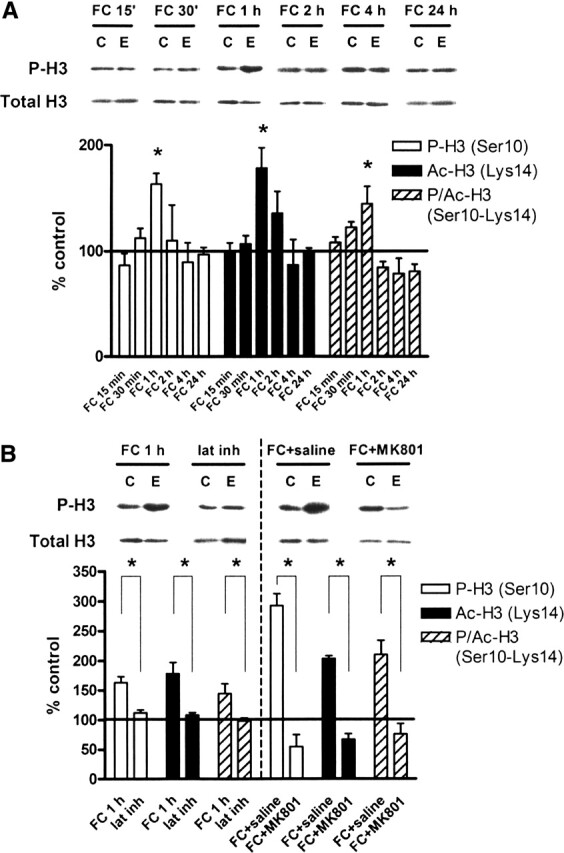
Contextual fear conditioning regulates histone H3 phosphorylation, acetylation, and phospho-acetylation in vivo. (A) Quantification of immunoblot densities for phospho-histone H3, acetyl-histone H3, and phospho-acetyl-histone H3 at different time points following contextual fear conditioning. Histone H3 phosphorylation, acetylation, and phospho-acetylation in area CA1 were significantly increased at 1 h after training (FC 1 h, n = 8) before returning to baseline and remaining even after 24 h. Total histone H3 was unchanged. Representative immunoblots for P-H3 and total H3 are shown for each time point. Control (C) samples appear on the left, and experimental (E) samples appear on the right. (B) Quantification of immunoblot densities for phospho-histone H3, acetyl-histone H3, and phospho-acetyl-histone H3. The latent inhibition paradigm (lat inh, n = 4) significantly reduced histone H3 phosphorylation, acetylation, and phospho-acetylation in area CA1 compared with fear conditioning (FC 1 h, n = 8). Injection of animals with MK801 (300 μg/kg) prior to fear conditioning (FC + MK801, n = 3) significantly reduced histone H3 phosphorylation, acetylation, and phospho-acetylation in area CA1 compared with injection with saline (0.9% NaCl, 1.25 mL/kg) prior to fear conditioning (FC + saline, n = 3). Total histone H3 was unchanged. Representative immunoblots for P-H3 and total H3 are shown for each condition. Control (C) samples appear on the left, and experimental (E) samples appear on the right. Error bars indicate standard error of the mean. Asterisks denote significant differences (P < 0.05) as determined by Tukey’s multiple comparison test.
