Abstract
Interleukin-12 (IL-12) enhances Th1-type T-cell responses and exerts antiangiogenic effects. We initiated a phase 1 pilot study of IL-12 in 32 patients with acquired immunodeficiency syndrome (AIDS)–related Kaposi sarcoma (KS) whose KS was progressing while on antiretroviral therapy. Fifteen patients had poor prognosis T1S1 disease. IL-12 was administered subcutaneously twice weekly at doses from 100 to 625 ng/kg. The maximum tolerated dose was 500 ng/kg, and the principal toxicities were flulike symptoms, transaminase or bilirubin elevations, neutropenia, hemolytic anemia, and depression. No tumor responses were seen at the lowest dose (100 ng/kg), but 17 of 24 evaluable patients at the higher doses had partial or complete responses (response rate, 71%; 95% confidence interval, 48%-89%). Only 3 of 17 patients had a change in antiretroviral therapy before responding, and there were no significant differences between responders and nonresponders with regard to changes in CD4 counts or viral loads. Patients had increases in their serum IL-12, interferon-γ, and inducible protein-10 (IP-10) after the first dose, and increases above baseline persisted after week 4. These results provide preliminary evidence that IL-12 has substantial activity against AIDS-related KS with acceptable toxicity and warrants further investigation for this indication.
Introduction
Kaposi sarcoma (KS), the most common tumor associated with human immunodeficiency virus (HIV) infection, is a multicentric angioproliferative disorder that frequently involves the skin.1,2 KS is caused by Kaposi sarcoma–associated herpesvirus (KSHV), also called human herpesvirus-8 (HHV-8), a gammaherpesvirus that contains several genes with angiogenic activity.3-5 The tumor cells of KS are spindle cells, and there is evidence that paracrine and autocrine stimulation of these cells by angiogenic and other factors is central to tumor pathogenesis.2,6 KS is not curable, and while some cases of acquired immunodeficiency syndrome (AIDS)–related KS respond to highly active antiretroviral therapy (HAART) or interferon alpha, long-term palliative cytotoxic therapy is often required.7-11 However, cumulative toxicity can limit the ability to continue otherwise effective therapy. The development of less toxic patient self-administered long-term therapy would thus be an important therapeutic advance.
Interleukin-12 (IL-12) is a heterodimeric glycoprotein cytokine that exerts a number of effects on T lymphocytes and natural killer (NK) cells.12-18 IL-12 can promote the development of Th1-type helper T cells, facilitate cytotoxic T-cell responses, enhance the NK-cell lytic activity, and induce production of interferon γ (IFN-γ). IL-12 showed antitumor activity in a number of animal models.19,20 It was subsequently shown that IL-12 can inhibit angiogenesis and that this effect was mediated through inducible protein 10 (IP-10) and monokine induced by interferon gamma (Mig).21-23 This ability of IL-12 to inhibit angiogenesis made it attractive to evaluate as treatment for KS. Also, it has been found that HIV-infected patients have reduced IL-12 production and that HIV-specific T-cell responses can be restored by IL-12 in ex vivo peripheral blood cells from these patients.24,25
Several phase 1 studies demonstrated that IL-12 could be administered to patients with acceptable toxicity.26-28 With this background, we initiated a phase 1 pilot study of IL-12 in patients with HIV-associated KS. The study was designed to test several doses of IL-12 because of the possibility that HIV-infected patients might react differently to this cytokine than patients with intact immune systems. The study also permitted the accrual of additional patients at the maximum tolerated dose to study the toxicity and activity profile of IL-12 in this disease.
Patients, materials, and methods
Study population
Patients 18 years or older with HIV and biopsy-proven KS were eligible if they had KS evaluable by noninvasive methods, more than a 3-month life expectancy, and Karnofsky performance status of 70 or greater. Patients had to be receiving a stable regimen of at least 2 anti-HIV drugs for 4 weeks prior to entry. Patients with life-threatening KS were ineligible. Patients could not have received specific anti-KS therapy within 3 weeks, pharmacologic doses of systemic glucocorticoids within at least 2 weeks, or either bone marrow–stimulating factors (except erythropoietin) or cytokines within 2 weeks of entry. Patients had to have a hemoglobin level of 90 g/L (9.0 g/dL) or higher, an absolute neutrophil count of 0.75 × 109/L (750 cells/mm3) or higher, and a platelet count of 75 × 109/L (75 000 cells/mm3) or higher. Patients had to have a total bilirubin level of 63.3 μM (3.7 mg/dL) or less, and were ineligible if they had an aspartate transaminase level of more than 2.5 times the upper limit of normal or history of hepatic cirrhosis. Additional exclusion criteria included recent severe infections or clinically significant autoimmune disease. The protocol and informed consent were approved by the National Cancer Institute institutional review board, and all patients gave written informed consent, in accordance with the Declaration of Helsinki.
Treatment regimen
This dose-escalating pilot/dose-finding study was conducted at the NIH Clinical Center, Bethesda, MD. IL-12 was provided by the Cancer Therapy Evaluation Program (CTEP) of the NCI through an agreement with the manufacturer, Genetics Institute, Cambridge, MA. Cohorts of 3 to 6 patients were to be successively entered and administered IL-12 subcutaneously twice weekly at the following doses: 100, 300, 500, 625, and 750 ng/kg. When a toxic dose (at which 2 or more patients developed dose-limiting toxicity within the first 4 weeks) was reached, up to 10 additional evaluable patients could be entered to receive the dose below that, considered the maximum tolerated dose (MTD). The protocol initially called for IL-12 to be administered by a health professional, but was later amended to permit self-administration. One 50% dose reduction was permitted for dose-limiting toxicities that resolved to grade 1 within 4 weeks while the drug was temporarily stopped. Patients were initially treated for up to 6 months as long as they did not meet criteria for leaving the study, which included dose-limiting toxicity, life-threatening infections, or progression of KS requiring cytotoxic chemotherapy. The protocol was subsequently amended to allow patients to receive IL-12 indefinitely as long as the study remained open and they did not meet criteria for leaving the study.
Evaluations of patients and response assessment
Patients were evaluated at entry, once weekly for the first 6 weeks, and every 4 weeks thereafter. History, physical examinations, and routine laboratory assessments were performed at each visit. Toxicity was graded using the NCI common toxicity criteria version 1.0.29 Patients were assigned at entry into good or poor prognostic KS groups based on tumor involvement at the time of most extensive disease (T), immunologic status (I), and systemic illness (S).30 Response assessments were made using a minor modification of the method developed by the AIDS Clinical Trial Group.31,32 Only lesions that had never received local therapy were assessed. Assessments of visible lesions were made every 4 weeks, and radiologic and photographic documentation was obtained when possible every 8 weeks as well as at the end of treatment.
Patients were considered evaluable for tumor response if they completed at least 8 weeks of therapy or progressed before that point. A partial response (PR) was defined as no progressive disease (PD) and at least a 4-week persistence of a 50% decrease in the sum of the cross products of 5 marker lesions identified at entry, 50% reduction in the total number of lesions, flattening of 50% or more of the nodular lesions, or 50% decrease in radiologically measurable visceral lesions. For patients with more than 50 lesions at entry, representative areas of the body were selected and assessed. A complete response (CR) was defined as the absence of any evident disease for 4 weeks with confirmation by biopsy. PD was defined as either an increase from baseline of 25% or more in the parameters used to define a PR or development of new or increasing tumor-related edema or effusion that interfered with the patient's normal activities. When possible, designation of progression was made when the criteria were met in 2 measurements spaced at least one week apart. Duration of response was defined as the interval between the first achievement of response and the documentation of PD. Stable disease was defined as disease that did not meet any of the criteria for other responses.
Analysis of immunologic and virologic parameters
Lymphocyte subsets were assessed by fluorescent-activated cell sorting.33 HIV-1 mRNA levels in plasma were measured by quantitative RNA polymerase chain reaction (Roche Amplicor HIV-1 Monitor Kits; Roche Diagnostic Systems, Branchburg, NJ); the lower limit of detection initially was 200 copies/mL, but after July 1999 was fewer than 50 copies/mL. Viral load measurements below the limits of detection with the less sensitive assay were subsequently repeated on stored plasma using the more sensitive test. Serum collected at entry and every 4 weeks was assessed in batches for levels of IL-12, IFN-γ, and IP-10 using enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN). Also, serum for the measurement of these parameters was drawn 18 hours after the first dose of IL-12, starting with the 300-ng/kg dose group. The baseline level on each patient (from a sample obtained before treatment) was compared with the median of the levels from samples on the same patient obtained between weeks 4 and 20.
Statistical analyses
Changes in CD4 counts, log10 viral load, and serum cytokine levels from entry to subsequent time points were determined by subtracting the appropriate paired values from one another, and the difference was tested for being significantly different from zero using the Wilcoxon signed rank test. For viral load measurements below the level of detection, the log 10 value of 50 (1.69) was used. Differences between absolute CD4 counts, changes in absolute CD4 counts, or log viral loads between groups of patients were evaluated using an exact Wilcoxon rank sum test. The Jonckheere-Terpstra test for trend was used to assess the significance of a difference in values of a continuous parameter according to an ordered category, such as clinical response.34 All P values are 2-sided and were not adjusted for multiple comparisons. Progression-free survival was calculated from the date of joining the study until the date of progression or the date patients were taken out of the study as appropriate. Progression-free survival was also calculated using clinical assessments made after the patient came off this study, as long as the patient did not receive other specific anti-KS therapy. The probability of progression-free survival was determined by the Kaplan-Meier method.
Results
Baseline patient demographic, virologic, and immunologic characteristics
Thirty-two male patients aged 27 to 51 years were enrolled between January 2, 1997, and October 10, 2001 (Table 1). Two patients originally enrolled at the 100-ng/kg dose were subsequently re-enrolled at a higher dose for a total of 34 enrollments. Three to 6 patients were entered per dose until the toxic dose was defined, and 11 additional patients were subsequently enrolled at the maximum tolerated dose (500 ng/kg). In all, 5 patients were enrolled at the 100-ng/kg dose, 8 at the 300-ng/kg dose, 17 at the 500-ng/kg dose (including the 2 re-enrollments), and 4 at the 625-ng/kg dose.
Table 1.
Characteristics of patients at entry
| Characteristic | Value |
|---|---|
| No. of patients (no. of enrollments*) | 32 (34) |
| Age, y | |
| Median | 39 |
| Range | 27-51 |
| CD4 cells/mm3 | |
| Median | 306 |
| Range | 33-745 |
| HIV viral load, log10 copies/mL | |
| Median | 2.70 |
| Range | 1.69-5.20 |
| Antiretroviral therapy, no. of patients | |
| HAART | 32 |
| Dual NRTI therapy | 2 |
| TS prognostic category, no. of patients | |
| Good (T0 or S0) | 19 |
| Poor (T1S1) | 15 |
| Extensive tumor (T1) | 27 |
| Advanced HIV disease (S1) | 18 |
| Other characteristics, no. of patients | |
| 150 or fewer CD4 cells/mm3 | 9 |
| Prior opportunistic infection | 12 |
| Resistant HIV† | 9 |
| More than 50 KS lesions | 26 |
| Prior KS therapy, no. of patients | |
| Radiation therapy | 5 |
| Systemic chemotherapy | 21 |
| Investigational therapy | 13 |
Two patients originally enrolled at 100 ng/kg were re-enrolled at a higher dose. Subsequent analyses refer to the 34 enrollments.
Resistant HIV was defined as an HIV viral load of more than 3.3 log10 copies/mL in a patient who had received multiple HIV regimens and was currently on an anti-HIV regimen.
At entry, all but 2 patients were on HAART, defined as a combination of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and either a protease inhibitor or a non–nucleoside reverse transcriptase inhibitor. The other 2 patients were receiving dual NRTI therapy. All patients had worsening of their KS on this stable antiretroviral regimen during the weeks just preceding their entry. All but 6 of the 32 patients had received specific prior anti-KS therapy. Nineteen had received systemic chemotherapy, and 13 had received prior investigational therapy. According to a modification of the AIDS Clinical Trials Group TIS staging system for KS,30 refined for lack of prognostic significance of CD4 cell level in the era of HAART,35 19 patients were assessed as being good risk (either T0 or S0) and 13 patients (15 enrollments), as poor risk (T1S1). All of the poor-risk patients had history of pulmonary or visceral KS or tumor-associated edema or extensive oral involvement and prior AIDS-related systemic illness. Of the good-risk patients, 13 had extensive cutaneous involvement (50 or more lesions). Two had CD4 cell counts of less than 150/mm3, and 3 had prior HIV-related illness.
The median entry CD4 cell count was 306 cells/mm3 (range, 33 to 745 cells/mm3), and the median entry HIV viral load was 2.70 log10 (range, < 1.69-5.20 log10). For those with T1S1 (poor risk) disease, the median entry CD4 cell count was 156 cells/mm3 (range, 33-506 cells/mm3), while for those patients with good risk, the median entry CD4 cell count was 364 cells/mm3 (range, 69 to 745 cells/mm3). The median HIV-1 viral load was similar between the TS-defined risk groups: 2.91 log10 copies mRNA/mL (range, < 1.69-4.99 log10) for the poor risk, and 2.61 log10 mRNA/mL (range, < 1.69-5.20 log10) for the good risk.
Treatment and outcome
Toxicity. Patients received IL-12 for a median of 5.5 months (range, 0 to 57.5 months). The principal laboratory and clinical toxicities observed are listed in Table 2. One of the 4 patients on the 625-ng/kg dose came off the study for fever and night sweats during the first 4 weeks, and 1 had to have his dosing stopped for toxicity (rash) at week 7; this was thus considered a toxic dose, and the 500-ng/kg dose the MTD.
Table 2.
Principal toxicities observed on IL-12
| Toxicity, grade | 100 ng/kg IL-12 | 300 ng/kg IL-12 | 500 ng/kg IL-12 | 625 ng/kg IL-12 |
|---|---|---|---|---|
| Laboratory toxicities | ||||
| Anemia without hemolysis | ||||
| Grade 3 | 0 | 1 | 0 | 0 |
| Anemia with hemolysis | ||||
| Grade 2 | 0 | 1 | 1 | 0 |
| Grade 3 | 0 | 0 | 1 | 0 |
| Grade 4 | 0 | 0 | 1 | 0 |
| Neutropenia | ||||
| Grade 3 | 1 | 2 | 6 | 2 |
| Grade 4 | 1 | 4 | 7 | 1 |
| Elevated bilirubin | ||||
| Grade 3 | 0 | 2 | 4 | 1 |
| Grade 4 | 0 | 0 | 1 | 0 |
| Elevated transaminase | ||||
| Grade 3 | 0 | 5 | 2 | 2 |
| Elevated amylase | ||||
| Grade 3 | 0 | 1 | 0 | 0 |
| Clinical toxicities | ||||
| Constitutional† | ||||
| Grade 2 | 2 | 3 | 10 | 2 |
| Grade 3 | 0 | 0 | 1 | 1 |
| Arthralgia | ||||
| Grade 2 | 0 | 0 | 3 | 2 |
| Depression | ||||
| Grade 1 | 0 | 0 | 3 | 1 |
| Grade 2 | 0 | 2 | 2 | 1 |
| Grade 5 | 0 | 1 | 0 | 0 |
| Headache | ||||
| Grade 2 | 0 | 2 | 4 | 2 |
| Grade 3 | 0 | 0 | 1 | 0 |
| Nausea | ||||
| Grade 2 | 0 | 0 | 2 | 2 |
| Vomiting | ||||
| Grade 2 | 0 | 0 | 2 | 1 |
| Skin rash | ||||
| Grade 3 | 0 | 0 | 0 | 1 |
| Weight loss | ||||
| Grade 3 | 0 | 1 | 0 | 0 |
Shown here are the principal toxicities and the grades observed in patients receiving IL-12 that were not primarily attributed to another cause. All patients were assessable for toxicity. For patients receiving 100 ng/kg, n = 5; 300 ng/kg, n = 8; 500 ng/kg, n = 17; and 625 ng/kg, n = 4. Shown are all toxicities of grade 3 and above. In addition, anemia with hemolysis and other noteworthy toxicities of grade 2 are shown, as well as any grade of depression. In addition to these toxicities, (1) 1 patient on the 100-ng/kg dose had grade 3 anemia attributable to zidovudine; (2) 3 patients had elevated bilirubin levels (1 grade 3 and 2 grade 4) attributable to indinavir administration (and in one case also to sepsis); (3) 1 patient on the 300-ng/kg dose died of sepsis arising in a site of KS 1 week after stopping dosing, and 1 patient on the 500-ng/kg dose died of complications from concurrent multicentric Castleman disease and antithrombotic therapy administered for a possible pulmonary embolism. In addition, one patient on the 500-ng/kg dose developed a grade 4 elevated bilirubin and transaminases, which on investigation were found to be attributable to his taking at least twice the prescribed dose.
*In addition to the anemia with hemolysis toxicities noted, one patient on the 500-ng/kg dose and one patient on the 625-ng/kg dose was found to have increased hemolysis without anemia.
Constitutional symptoms observed were fevers, myalgias, and fatigue.
The most frequent laboratory toxicities of grade 3 or higher were neutropenia, elevated bilirubin, and elevated transaminase levels (Table 2). One patient on the 500-ng dose developed grade 4 elevations of bilirubin and hepatic transaminases; on questioning, he had been self-administering himself at least twice the intended amount of IL-12. These abnormalities improved upon discontinuing the IL-12. Four patients developed anemia with hemolysis of grade 2 or higher, and an additional patient developed hemolysis without anemia. One patient with hemolysis on the 500-ng/kg dose was found to have parvovirus B19 infection; he recovered with temporary stopping of IL-12 and intravenous immunoglobulin therapy, and was then restarted on IL-12 without difficulties. In each of the other cases, the anemia with hemolysis resolved upon stopping the IL-12.
The most common clinical toxicities were low-grade flulike symptoms characterized by fevers, chills, fatigue, headache, arthralgias, and myalgias (Table 2). Overall, grade 3 constitutional symptoms occurred in one patient each at the 500-ng/kg and 625-ng/kg doses, and grade 2 constitutional symptoms occurred in a total of 17 patients, most frequently in those on the 500- and 625-ng/kg doses. Other relatively frequent clinical toxicities of grade 2 or above and at least possibly related to IL-12 included arthralgia (5 patients), depression (6 patients), headache (9 patients), nausea (4 patients), vomiting (3 patients), skin rash (1 patient), and weight loss (1 patient) (Table 2). Overall, depression of any grade worsened or was noted in 10 patients while on IL-12. While none of these patients appeared to be depressed at entry, 8 patients were on antidepressant medication, and an additional patient had a history of depression. Because most patients had pre-existing depression, it was not initially linked to IL-12 administration; however, a possible relationship to the drug became more apparent when several patients reported mood improvement after IL-12 was stopped. In one patient, depression was accompanied by paranoid thoughts. One patient on the 300-ng/kg dose level died from suicide after 63 weeks on IL-12. He was on antidepressant medication at entry, and multiple illicit drug use was uncovered during investigation of his death. The illicit drug use was thought to be a major factor leading to the suicide, although a contribution of IL-12 to his depression could not be absolutely discounted. Two other patients died from apparent complications of their disease or other therapies. One died of sepsis arising in a site of KS; he had received paclitaxel after stopping IL-12 and was neutropenic when he died. The other died of complications of concurrent Castleman disease (Table 2). These were the only 3 deaths from any cause in the study. Arthralgia and headaches were generally most pronounced after the first few doses of IL-12 and largely resolved after this time. At the doses below the MTD, a total of 4 patients left the study for toxicity: 1 patient each at the 300-ng/kg and 500-ng/kg doses for hepatic transaminitis elevations and 2 at the 500-ng/kg dose for hemolytic anemia. Other than these patients, the drug was generally well tolerated at doses up to 500 ng/kg except for mild constitutional symptoms during the first 2 or so weeks of dosing.
KS tumor response. Of the 34 patients who entered, 28 were evaluable for tumor responses. Of these, there were 17 major responses (4 pathologically confirmed CRs and 13 PRs) for an overall response rate of 61% (95% confidence interval [CI], 41% to 78%; Table 3). It should be noted that one of the patients scored as a CR had some residual edema in his lower extremities that was thought to be related to prior extensive radiotherapy of this region. None of the 5 patients who received the 100-ng/kg dose had responses, and this dose did not appear to be active. Considering the next 2 doses, 300 ng/kg and 500 ng/kg, which were tolerable for long-term dosing, 15 of 21 evaluable patients responded for an overall response rate of 71% (95% CI, 48% to 89%). The response rate in the 3 evaluable patients at the highest (625 ng/kg) dose was essentially the same (67%) as that seen at the previous 2 doses, and for subsequent analyses of active doses, this dose will be included for completeness. This results in a total of 17 responses in 24 patients (71%) treated on the 300- to 625-ng/kg dose levels (95% CI, 49% to 87%). Finally, taking all patient entries on an intent-to-treat basis, major responses were seen in 17 of 34 entries for a response rate of 50% (95% CI, 32% to 68%). This trial underwent a response review conducted by CTEP on December 11, 2000, and the responses were confirmed.
Table 3.
KS tumor responses by dose of IL-12
| IL-12, ng/kg | No. of patients | No. of evaluable patients | CR | PR | SD | PD | PR + CR (% of evaluable patients) |
|---|---|---|---|---|---|---|---|
| 100 | 5 | 4 | 0 | 0 | 0 | 4 | 0 (0) |
| 300 | 8 | 7 | 1 | 5 | 0 | 1 | 6 (86) |
| 500 | 17 | 14 | 3 | 6 | 3 | 2 | 9 (64) |
| 625 | 4 | 3 | 0 | 2 | 0 | 1 | 2 (67) |
| 300 to 625 | 29 | 24 | 4 | 13 | 3 | 4 | 17 (71) |
| Total | 34 | 28 | 4 | 13 | 3 | 8 | 17 (61) |
KS responses to IL-12 analyzed by dose administered.
Numbers in parentheses are the major responses (partial plus complete responses) expressed as a percentage of evaluable patients.
CR indicates complete response; PR, partial response; SD, stable disease; and PD, progressive disease.
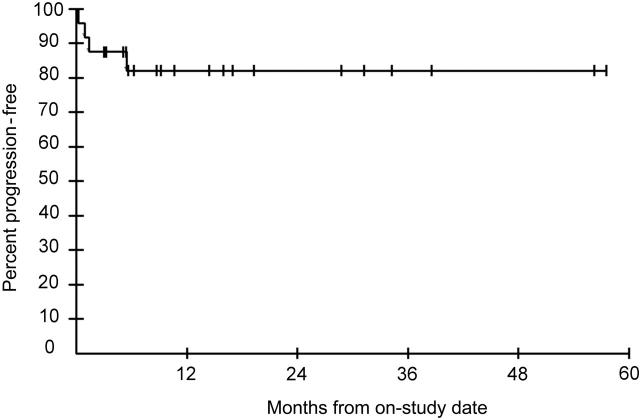
The median time to first response (partial or complete) was 18 weeks (range, 6 to 56 weeks). Of interest, 3 patients who responded first had tumor progression, but according to the protocol were able to continue IL-12 therapy; 1 of these 3 went on to have a CR. The 4 patients who attained a CR did so at 68, 169, 243, and 253 weeks after entry; these patients then had their IL-12 stopped within 6 weeks of attaining a CR. For the evaluable patients at the doses thought to be active (300 ng/kg and higher), the median progression-free survival was not reached, although the probability of progression-free survival at 4 years was 82% (Figure 1). After completing the study, the majority of patients were followed in the NCI clinic. If this poststudy period is included and patients were censored when they received additional KS therapy (exclusive of HAART) or were lost to follow-up, the probabilities of progression-free survival were 77% at 36 months, 67% at 60 months, and 50.5% at 80 months. Of the 4 patients with a CR, 2 continued HAART and had a sustained CR with no further anti-KS therapy when last seen 15 and 34 months after the IL-12 was stopped. Two of the patients who had a CR decided to stop their HAART, and these patients both had recurrence of their KS 42 and 43 months after their CR. Overall, there were no clear differences in the response rate or the duration of the overall response among patients on the 300-, 500-, or 625-ng/kg doses.
Figure 1.
Progression-free survival in months of evaluable patients treated with IL-12 at a dose of 300 ng/kg or higher.
Of the 28 patients evaluable for response, 13 were poor risk (T1S1) and 15 were good risk (T0 or S0). Overall, 11 (73%) of 15 good-risk patients had a major response, while 6 (46%) of 13 poor-risk patients responded (Table 4). The median entry CD4 count of those who responded with a PR or CR was higher than those who failed to respond (median, 370 cells/mm3 vs 191 cells/mm3; P = .026). In addition, the median entry log10 viral load (particles/mL) was slightly lower in the responders compared with the nonresponders. (1.75 vs 3.66, P = .04) Considering patients at all dose levels, there was no difference in the fraction of evaluable patients who responded between those who did or did not have previous anti-KS therapy (13/23 responses in patients with prior therapy vs 4/5 in those without; P = .62 by 2-tailed Fisher exact test).
Table 4.
KS risk group and HAART therapy in patients with KS responses
| Patient no. | IL-12, ng/kg | Tumor risk category (T)* | Symptom risk category (S) | HAART at entry, wk | Weeks to first PR | HAART at PR, wk | ART change prior to response |
|---|---|---|---|---|---|---|---|
| 1 | 300 | 1 | 0 | 12 | 52 | 64 | No |
| 2 | 300 | 1 | 0 | 87 | 18 | 105 | No |
| 3 | 300 | 1 | 1 | NA† | 12 | NA† | No |
| 4 | 300 | 1 | 1 | 44 | 16 | 60 | No |
| 5 | 300 | 1 | 1 | 14 | 8 | 21 | No |
| 6 | 300 | 1 | 1 | 207 | 36 | 243 | Yes‡ |
| 7 | 500 | 1 | 0 | 23 | 56 | 79 | No |
| 8 | 500 | 1 | 0 | 113 | 52 | 165 | No |
| 9 | 500 | 1 | 0 | 12 | 8 | 20 | No |
| 10 | 500 | 1 | 0 | 169 | 36 | 205 | No |
| 11 | 500 | 1 | 0 | 38 | 26 | 64 | Yes‡ |
| 12 | 500 | 1 | 0 | 16 | 8 | 24 | No |
| 13 | 500 | 0 | 0 | 82 | 16 | 98 | Yes‡ |
| 14 | 500 | 1 | 1 | 113 | 28 | 141 | No |
| 15 | 500 | 1 | 1 | 78 | 6 | 84 | No |
| 16 | 625 | 0 | 0 | 142 | 24 | 166 | No |
| 17 | 625 | 0 | 0 | 176 | 12 | 188 | No |
HAART indicates highly active antiretroviral therapy; PR, partial response; ART, antiretroviral therapy; and NA, not applicable.
Of the patients who were scored as T1, 8 had edema of the legs or feet, 1 had ulcerative KS lesions of the feet, 1 had pulmonary KS, 1 had oral KS, and 4 had a history of oral KS (1 patient) or visceral KS (3 patients, 1 each with pulmonary, gastrointestinal, and extensive oral involvement).
Patient 3 had been on dual nucleoside anti-HIV therapy with stavudine and lamivudine for 43 weeks at entry and remained on this therapy until he had a PR at week 12.
Patient 6 had his dose of saquinavir increased 2 weeks after entry and then changed to fortovase; KS response patient 11 had his HAART regimen changed 5 weeks before he had a PR; KS response patient 13 had the zidovudine in his HAART regimen changed to stavudine because of anemia 4 weeks before he had a PR.
All the patients who responded had been on a stable antiviral regimen for at least 12 weeks, and in each case the KS was worsening on this regimen in the weeks prior to entry. Information on the antiretroviral therapy in the 17 patients who had major tumor responses (partial or complete) is provided in Table 4. All but one had been on HAART for at least 12 weeks prior to entry, and 9 had been on HAART for more than a year. Moreover, 13 of the 17 patients had been on HAART for more than a year at the time they had a tumor response, and only 3 of the patients who responded had any change in their antiretroviral therapy prior to response (Table 4).
Relationship of tumor response to CD4 count and viral load change. KS responses to antiretroviral therapy appear to be mediated in part through effects on HIV viral load and CD4 counts. To further study the possible influence of antiretroviral therapy on the KS responses, we analyzed the CD4 counts and viral loads. We considered the changes in these parameters between entry and either week 12 or the time of tumor response (or last time in the study if patients had stable disease). When considering all evaluable patients, there was a small drop in the median absolute CD4 count from entry (median, 306 cells/mm3) to week 12 of 46 cells/mm3 (P = .005, Wilcoxon signed rank test) and from entry to time of response of 72.5 cells/mm3 (P = .032). However, there was no change in the log10 viral load from entry (median, 2.51) to either week 12 (median change, 0.0; P = .93) or the time of response (median change, 0.0; P = .81). Comparing the patients who had either a PR or a CR to those who did not respond, there was no significant difference in the change in CD4 cells at week 12 (median change, –46 cells/mm3 for responders vs –49 cells/mm3 for nonresponders; P = .999). Also, there was no difference in the change in log 10 viral load at week 12 (median change, 0.0 for both groups; P = .96). At the time of response, there was a median –104 cell/mm3 decrease in CD4 count of responders and a median –2 cell/mm3 decrease in patients without a major response (P = .22). Finally, neither the responders nor the nonresponders had a significant change in their median viral load at the time of response (median change, 0.0 for responders and 0.13 for nonresponders), and there was no significant difference between the groups (P = .94).
Restricting the analysis to the apparent active doses of 300 ng/kg or higher, there was a trend toward a decrease in the absolute CD4 count during IL-12 therapy (P = .053). Table 5 summarizes the tumor responses in evaluable patients who received 300 ng/kg or more IL-12 categorized by the change in CD4 count from entry to the time of response. By an exact Jonckheere-Terpstra trend test, there was no significant association between the change in CD4 count and the response rate (P = .22). Also, comparing responders with nonresponders at the 500-ng/kg dose level (the only dose level with adequate subjects to do the comparison), an exact Wilcoxon rank sum test showed that there was no association between response and CD4 change (P = .44).
Table 5.
Relationship between KS tumor response and change in CD4 count
|
Response, no. (%)
|
||||
|---|---|---|---|---|
| Response vs entry: change in CD4 count, cells/mm3 | No. of evaluable patients | Partial response | Stable disease | Progressive disease |
| Within 50 of entry | 6 | 1 (17) | 0 (0) | 5 (83) |
| Decrease by more than 50 | 16 | 12 (75) | 1 (6) | 3 (19) |
| Increase by more than 50 | 6 | 4 (67) | 2 (33) | 0 (0) |
| Increase by more than 100 | 3 | 3 (67) | 1 (33) | 0 (0) |
Depicted are patients who received 300 ng/kg or more IL-12 and were evaluable for tumor response. Change in CD4 cell count is calculated from the time of entry until the time that patients achieved a tumor response (partial response, progressive disease, or stable disease). Patients with stable disease are assessed at the time they left the study. Patients who went on to develop a complete response are assessed at the time of their first partial response.
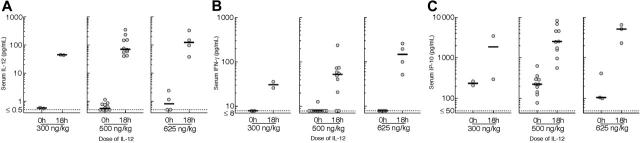
Serum levels of IL-12, IFN-γ, and IP-10. Serum levels of IL-12, IFN-γ, and IP-10 were measured at baseline and 18 hours after the first dose in patients entered at 300 ng/kg or higher when possible. Administration of IL-12 resulted in an increase in each of these parameters (Figure 2). Moreover, there was a disproportionate increase in the median level of each of these parameters once the 500-ng/kg dose of IL-12 was reached; in particular, the increase from 500 ng/kg to 625 ng/kg IL-12 resulted in more than a doubling of the median serum concentrations of both IFN-γ (from 51 pg/mL to 146 pg/mL) and IP-10 (from 2500 pg/mL to 5053 pg/L).
Figure 2.
Serum levels of IL-12, IFN-γ, and IP-10. Shown are serum levels of (A) IL-12, (B) IFN-γ, or (C) IP-10 at entry and 18 hours after the first dose of IL-12 in patients receiving 300 ng/kg, 500 ng/kg, or 625 ng/kg IL-12. Each point depicts a serum concentration in a single patient; the lines show the median values. The dotted lines depict the lower limit of detection for each assay.
Serum samples were also obtained during clinic visits every 4 weeks up to week 24. Assay of these samples showed that IL-12, IFN-γ, and IP-10 levels measured at a given time point after a dose of IL-12 decreased somewhat during the first few weeks of dosing but then remained above baseline at relatively constant levels. For the patients receiving 500 ng/kg, for example, samples obtained after week 4 were drawn a median of 77 hours after the last dose of IL-12. Comparing these samples to the baseline values for each patient, there was an increase in median serum IL-12 from 0.67 at baseline to 24.4 pg/mL after week 4 (P = .001). Also, there was an increase in median serum IFN-γ from less than 8 pg/mL to 18.5 pg/mL after week 4 (P = .002) and in IP-10 from 223 pg/mL to 444 pg/mL after week 4 (P = .016).
Discussion
In this paper, we show that IL-12 given subcutaneously twice weekly could be administered for up to 57 months in patients with AIDS-associated KS who were receiving antiretroviral therapy and that the MTD in this population was 500 ng/kg twice weekly. Moreover, we show that while no responses were seen at the 100-ng/kg dose, 71% of evaluable patients receiving 300 or 500 ng/kg IL-12 had a major (partial or complete) KS tumor response. Finally, we show that IL-12 administration to this patient population resulted in persistent increases in measurable serum IFN-γ as well as IP-10.
Administration of HAART has been reported to induce KS tumor responses in a subset of patients with HIV-associated KS.36-38 This raises the question as to whether the responses were due to IL-12 administration or antiretroviral therapy. Several factors make it unlikely that they were simply due to HAART. First, patients were required to be on a stable regimen of antiretroviral therapy and all had KS that was worsening on their admission regimen. In addition, the vast majority of patients who responded had been on HAART for more than a year at the time of response and only 3 had any change in their antiretroviral therapy before responding. In this study, there was a trend downward in the CD4 counts of patients who responded, and no difference in the change in CD4 counts between responders and nonresponders. By contrast, patients whose KS has been reported as responding to HAART generally had increases in their CD4 cells.36 Finally, 14 patients who responded in the current study had advanced KS scored as T1 (Table 4). This is noteworthy in light of a recent survey of the entire KS literature, in which only 5 patients with T1 disease were identified who responded to HAART alone.37 Finally, there appeared to be a dose relationship in the response to IL-12, with no responses observed at the lowest (100 ng/kg) dose tested. Taken together, these factors lend credence to the argument that IL-12 was a substantial contributor to the responses in this study.
Preclinical studies performed with IL-12 suggested that this agent may hold promise for the treatment of a wide variety of tumors.12,16-23 However, in initial phase 1 studies and phase 2 clinical trials in a variety of solid tumors, IL-12 generally showed little activity.26,28,39-41 Perhaps the most promising results prior to this study were seen in studies in non-Hodgkin lymphoma and in cutaneous T-cell lymphoma.42,43 Also, a relatively high (69%) response rate was observed in a phase 1 trial of IL-12 in combination with rituximab in patients with B-cell lymphoma.44
The results of the present study suggest that of the tumors studied to date, KS is the most responsive to IL-12. What factors may account for the high level of activity? One is that the spindle cells in KS lesions are derived from endothelial cells, and the causative virus, KSHV, encodes for a number of factors that induce angiogenesis.2,4,5,45-48 KS may thus be particularly responsive to antiangiogenic approaches. Moreover, IL-12 has been shown to help protect against other herpesvirus infections in animal models.49 Cells from patients with HIV infection produce less IL-12 ex vivo than cells from healthy controls, and addition of IL-12 has been shown to correct certain immune responses in T cells from HIV-infected individuals.24,25 While studies of relatively short-term (4 weeks or less) IL-12 administration to HIV-infected patients did not show improvement in immunologic factors,50,51 such improvement may have occurred over the longer treatment periods used in the present study or may have involved subtle immune functions. Indeed, the tendency of KS to occur in patients with low CD4 cells or respond to HAART-induced CD4 increases suggests that this tumor is responsive to immunologic control.36-38,52 A final mechanism by which IL-12 may work in KS is through inhibition of a KSHV-encoded protein, the product of ORF74. The G-protein–coupled receptor that is encoded by ORF74 is constitutively active, induces production of VEGF and an angiogenic state, and has been shown to induce KS-like lesions in animal models.48,53,54 IP-10 down-regulates the activity of this receptor at concentrations attained on this trial, and this may contribute to the activity of IL-12 in KS.55
Assays of serum IL-12, IFN-γ, and IP-10 showed that these factors were all increased 18 hours after the initial dose of IL-12. Eighteen hours was chosen because previous pharmacokinetic studies suggested it was near the peak of IL-12 levels.28,44,50,56 As previously reported, increases in the dose of IL-12 resulted in a disproportionate increase in the serum levels of IL-12 at the higher doses50; in the present study, a similar disproportionate increase was also seen in serum levels of IFN-γ and IP-10. Moreover, this study demonstrates that IL-12 can yield substantial increases in IFN-γ and IP-10 even in immunosuppressed patients with AIDS-KS. In a previous study of IL-12 in patients with HIV infection, no sustained increase in serum IFN-γ levels was seen.51 By contrast, in the present study, serum levels of IFN-γ, in addition to IL-12 and IP-10, continued to be increased above baseline in samples obtained after week 4. This result is consistent with IL-12 having a biologic effect on KS throughout the study.
Patients with AIDS-KS are sometimes particularly sensitive to drug toxicity. The maximum tolerated dose of IL-12 on this study was 500 ng/kg, and the toxicity profile was similar to that in other tumors.26,28,39-44 Some patients were able to tolerate IL-12 for more than 4 years, a substantially longer time than studied in other trials. Also, consistent with previous studies, constitutional symptoms tended to abate after the first 2 doses; to ameliorate these effects in future studies, it may be worthwhile to use the 300-ng/kg dose and perhaps lower doses for the first 2 weeks. One noteworthy toxicity in this study was depression. Nearly all of these patients had pre-existing depression, and it is difficult to tease out the exact role of IL-12. By contrast to this study, depression has been observed only rarely in other cancer trials of IL-12.43 Patients with AIDS-KS may be particularly susceptible to depression, and we similarly observed a high incidence in a recent trial of thalidomide in this population.57 Of interest, patients with major depression have been reported to have elevated levels of serum IL-12.58 While the results here may in part reflect pre-existent depression in the subjects, patients should be carefully monitored for depression in future trials of IL-12 in other diseases.
IL-12 has been shown to enhance replication of HIV in T cells, although this could easily be blocked with nucleoside anti-HIV drugs.59 Patients in the study received anti-HIV therapy, and we did not observe any increase in the HIV viral load. Additional concerns were that there is some laboratory evidence that IFN-γ may enhance replication of KSHV in peripheral blood mononuclear cells60,61 and that no anti-KS activity had been seen in 2 small trials of partial purified IFN-γ conducted before the development of effective anti-HIV therapy.62,63 In spite of these concerns, IL-12 appeared to be active in a high percentage of the patients with KS. As noted previously, several factors make it unlikely that these responses were simply from the administration of HAART. Nonetheless, a randomized trial will be needed to demonstrate conclusively that IL-12 has potent anti-KS activity. Given its apparent activity in KS, its tolerability, and lack of bone marrow suppression, such a trial is warranted.
Acknowledgments
The authors thank the patients who volunteered for this study; Dr James Zwiebel and other members of the Cancer Therapeutics Evaluation Program, NCI; Dr Matthew Sherman of Genetics Institute; Dr Rachel Humphrey, Dr Lauri Welles, Jill Lietzau, CAPT Florentino Merced-Galindez, Karen Aleman, and Dr Betsy Read-Cannole of the HIV and AIDS Malignancy Branch; Dr Ellen Feigal of the NCI; Randy Stevens, Michael Baseler, and David Waters of Science Applications International; the data management team of the HIV and AIDS Malignancy Branch; the medical staff of the Medical Oncology Clinical Research Units of the NCI; and the nursing, pharmacy, social work, and medical staff of the National Institutes of Health Clinical Center.
Prepublished online as Blood First Edition Paper, February 28, 2006; DOI 10.1182/blood-2005-11-4455.
Supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute.
R.F.L. performed research, analyzed data, and cowrote the paper; J.M.P. designed research, analyzed data, and performed research; K.M.W. performed research and analyzed data; I.R.R.-C. performed research; G.T. contributed vital new technology and performed research; A.T.C. performed research; S.M.S. analyzed data; and R.Y. designed research, performed research, analyzed data, and cowrote the paper.
Several of the coauthors (R.F.L., J.M.P., K.M.W., G.T., and R.Y.) are listed as coinventors on patents related to the use of IL-12 to treat Kaposi sarcoma, and one of the authors (R.Y.) is listed as a coinventor on one or more US government patents involving dideoxycytidine (zalcitabine), dideoxyadenosine, dideoxyinosine (didanosine), zidovudine, and/or acyclovir as treatments for HIV infection. These inventions were made as full-time employees of the US government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to these patents have been assigned to the US government. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (Public Law 99-502).
The publisher or recipient acknowledges the right of the U.S. government to retain a nonexclusive, royalty-free licence in and to any copyright covering the article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342: 1027-1038. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff C, Weiss R. AIDS-related malignancies. Nat Rev Cancer. 2002;2: 373-382. [DOI] [PubMed] [Google Scholar]
- 3.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332: 1181-1185. [DOI] [PubMed] [Google Scholar]
- 4.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274: 1739-1744. [DOI] [PubMed] [Google Scholar]
- 5.Boshoff C, Endo Y, Collins PD, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278: 290-294. [DOI] [PubMed] [Google Scholar]
- 6.Ensoli B, Nakamura S, Salahuddin SZ, et al. AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243: 223-226. [DOI] [PubMed] [Google Scholar]
- 7.Groopman JE, Gottlieb MS, Goodman J, et al. Recombinant alpha-2 interferon for Kaposi's sarcoma associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984;100: 671-676. [DOI] [PubMed] [Google Scholar]
- 8.Krown SE, Real FX, Cunningham-Rundles S, et al. Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi's sarcoma. N Engl J Med. 1983;308: 1071-1076. [DOI] [PubMed] [Google Scholar]
- 9.Gill PS, Miles SA, Mitsuyasu RT, et al. Phase I AIDS Clinical Trials Group (075) study of adriamycin, bleomycin and vincristine chemotherapy with zidovudine in the treatment of AIDS-related Kaposi's sarcoma. AIDS. 1994;8: 1695-1699. [DOI] [PubMed] [Google Scholar]
- 10.Gill PS, Tulpule A, Espina BM, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi's sarcoma. J Clin Oncol. 1999;17: 1876-1883. [DOI] [PubMed] [Google Scholar]
- 11.Yarchoan R, Tosato G, Little RF. Therapy insight: AIDS-related malignancies: the influence of antiviral therapy on pathogenesis and management. Nature Clin Prac Oncology. 2005;2: 406-415. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170: 827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stern AS, Podlaski FJ, Hulmes JD, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990;87: 6808-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott P, Kaufman SHE. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12: 346-348. [DOI] [PubMed] [Google Scholar]
- 15.Brunda MJ. Interleukin-12. J Leukoc Biol. 1994;55: 280-288. [DOI] [PubMed] [Google Scholar]
- 16.Chan SH, Perussia B, Gupta JW, et al. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173: 869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993;151: 2444-2452. [PubMed] [Google Scholar]
- 18.Robertson MJ, Soiffer RJ, Wolf SF, et al. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175: 779-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunda MJ, Luistro L, Warrier RR, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178: 1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153: 1697-1706. [PubMed] [Google Scholar]
- 21.Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87: 581-586. [DOI] [PubMed] [Google Scholar]
- 22.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182: 155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87: 3877-3882. [PubMed] [Google Scholar]
- 24.Clerici M, Lucey DR, Berzofsky JA, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262: 1721-1724. [DOI] [PubMed] [Google Scholar]
- 25.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174: 46-53. [DOI] [PubMed] [Google Scholar]
- 26.Gollob JA, Mier JW, Veenstra K, et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Clin Cancer Res. 2000;6: 1678-1692. [PubMed] [Google Scholar]
- 27.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3: 409-417.9815699 [Google Scholar]
- 28.Motzer RJ, Rakhit A, Schwartz LH, et al. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res. 1998;4: 1183-1191. [PubMed] [Google Scholar]
- 29.Wittes RE. Common toxicity criteria for cancer clinical trials. In: Wittes RE, ed. Manual of Oncologic Therapeutics. Philadelphia, PA: J. B. Lippencott; 1991: 445-448.
- 30.Krown SE, Testa MA, Huang J. AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification: AIDS Clinical Trials Group Oncology Committee. J Clin Oncol. 1997;15: 3085-3092. [DOI] [PubMed] [Google Scholar]
- 31.Krown SE, Metroka C, Wernz JC. Kaposi's sarcoma in the acquired immunodeficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. J Clin Oncol. 1989;7: 1201-1207. [DOI] [PubMed] [Google Scholar]
- 32.Little RF, Merced-Galindez F, Staskus K, et al. A pilot study of cidofovir in patients with Kaposi's sarcoma. J Infect Dis. 2003;187: 149-153. [DOI] [PubMed] [Google Scholar]
- 33.Jankelevich S, Mueller BU, Mackall CL, et al. Long-term virologic and immunologic responses in human immunodeficiency virus type 1-infected children treated with indinavir, zidovudine, and lamivudine. J Infect Dis. 2001;183: 1116-1120. [DOI] [PubMed] [Google Scholar]
- 34.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2nd ed. New York, NY: John Wiley and Sons; 1999.
- 35.Nasti G, Talamini R, Antinori A, et al. AIDS-related Kaposi's sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS Clinical Trial Group Staging System in the HAART Era: the Italian Cooperative Group on AIDS and Tumors and the Italian Cohort of Patients Naive From Antiretrovirals. J Clin Oncol. 2003;21: 2876-2882. [DOI] [PubMed] [Google Scholar]
- 36.Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients: CISIH 92: Centre d'information et de soins de l'immunodeficience humaine. AIDS. 2000;14: 987-993. [DOI] [PubMed] [Google Scholar]
- 37.Krown SE. Highly active antiretroviral therapy in AIDS-associated Kaposi's sarcoma: implications for the design of therapeutic trials in patients with advanced, symptomatic Kaposi's sarcoma. J Clin Oncol. 2004;22: 399-402. [DOI] [PubMed] [Google Scholar]
- 38.Noy A, Scadden DT, Lee J, et al. Angiogenesis inhibitor IM862 is ineffective against AIDS-Kaposi's sarcoma in a phase III trial, but demonstrates sustained, potent effect of highly active antiretroviral therapy: from the AIDS Malignancy Consortium and IM862 Study Team. J Clin Oncol. 2005;23: 990-998. [DOI] [PubMed] [Google Scholar]
- 39.Hurteau JA, Blessing JA, DeCesare SL, Creasman WT. Evaluation of recombinant human interleukin-12 in patients with recurrent or refractory ovarian cancer: a gynecologic oncology group study. Gynecol Oncol. 2001;82: 7-10. [DOI] [PubMed] [Google Scholar]
- 40.Lenzi R, Rosenblum M, Verschraegen C, et al. Phase I study of intraperitoneal recombinant human interleukin 12 in patients with Mullerian carcinoma, gastrointestinal primary malignancies, and mesothelioma. Clin Cancer Res. 2002;8: 3686-3695. [PubMed] [Google Scholar]
- 41.Wadler S, Levy D, Frederickson HL, et al. A phase II trial of interleukin-12 in patients with advanced cervical cancer: clinical and immunologic correlates: Eastern Cooperative Oncology Group study E1E96. Gynecol Oncol. 2004;92: 957-964. [DOI] [PubMed] [Google Scholar]
- 42.Younes A, Pro B, Robertson MJ, et al. Phase II clinical trial of interleukin-12 in patients with relapsed and refractory non-Hodgkin's lymphoma and Hodgkin's disease. Clin Cancer Res. 2004;10: 5432-5438. [DOI] [PubMed] [Google Scholar]
- 43.Rook AH, Wood GS, Yoo EK, et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood. 1999;94: 902-908. [PubMed] [Google Scholar]
- 44.Ansell SM, Witzig TE, Kurtin PJ, et al. Phase 1 study of interleukin-12 in combination with rituximab in patients with B-cell non-Hodgkin lymphoma. Blood. 2002;99: 67-74. [DOI] [PubMed] [Google Scholar]
- 45.Folpe AL, Veikkola T, Valtola R, Weiss SW. Vascular endothelial growth factor receptor-3 (VEGFR-3): a marker of vascular tumors with presumed lymphatic differentiation, including Kaposi's sarcoma, kaposiform and Dabska-type hemangioendotheliomas, and a subset of angiosarcomas. Mod Pathol. 2000;13: 180-185. [DOI] [PubMed] [Google Scholar]
- 46.Skobe M, Brown LF, Tognazzi K, et al. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J Invest Dermatol. 1999;113: 1047-1053. [DOI] [PubMed] [Google Scholar]
- 47.Chang Y, Cesarman E, Pessin M, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266: 1865-1869. [DOI] [PubMed] [Google Scholar]
- 48.Bais C, Santomasso B, Coso O, et al. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391: 86-89. [DOI] [PubMed] [Google Scholar]
- 49.Matsuo R, Kobayashi M, Herndon DN, Pollard RB, Suzuki F. Interleukin-12 protects thermally injured mice from herpes simplex virus type 1 infection. J Leukoc Biol. 1996;59: 623-630. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson MA, Hardy D, Connick E, Watson J, DeBruin M. Phase 1 trial of a single dose of recombinant human interleukin-12 in human immunodeficiency virus-infected patients with 100-500 CD4 cells/microL. J Infect Dis. 2000;182: 1070-1076. [DOI] [PubMed] [Google Scholar]
- 51.Jacobson MA, Spritzler J, Landay A, et al. A Phase I, placebo-controlled trial of multi-dose recombinant human interleukin-12 in patients with HIV infection. Aids. 2002;16: 1147-1154. [DOI] [PubMed] [Google Scholar]
- 52.Rabkin CS, Biggar RJ, Horm JW. Increasing incidence of cancers associated with the human immunodeficiency virus epidemic. Int J Cancer. 1991;47: 692-696. [DOI] [PubMed] [Google Scholar]
- 53.Sodhi A, Montaner S, Patel V, et al. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60: 4873-4880. [PubMed] [Google Scholar]
- 54.Montaner S, Sodhi A, Molinolo A, et al. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3: 23-36. [DOI] [PubMed] [Google Scholar]
- 55.Geras-Raaka E, Varma A, Ho H, Clark-Lewis I, Gershengorn MC. Human interferon-gamma-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J Exp Med. 1998;188: 405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Portielje JE, Lamers CH, Kruit WH, et al. Repeated administrations of interleukin (IL)-12 are associated with persistently elevated plasma levels of IL-10 and declining IFN-gamma, tumor necrosis factor-alpha, IL-6, and IL-8 responses. Clin Cancer Res. 2003;9: 76-83. [PubMed] [Google Scholar]
- 57.Little RF, Wyvill KM, Pluda JM, et al. Activity of thalidomide in AIDS-related Kaposi's sarcoma. J Clin Oncol. 2000;18: 2593-2602. [DOI] [PubMed] [Google Scholar]
- 58.Kim YK, Suh IB, Kim H, et al. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. 2002;7: 1107-1114. [DOI] [PubMed] [Google Scholar]
- 59.Foli A, Saville MW, Baseler MW, Yarchoan R. Effects of the Th1 and Th2 stimulatory cytokines interleukin-12 and interleukin-4 on human immunodeficiency virus replication. Blood. 1995;85: 2114-2123. [PubMed] [Google Scholar]
- 60.Monini P, Colombini S, Sturzl M, et al. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood. 1999;93: 4044-4058. [PubMed] [Google Scholar]
- 61.Chang J, Renne R, Dittmer D, Ganem D. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology. 2000;266: 17-25. [DOI] [PubMed] [Google Scholar]
- 62.Krigel RL, Odajnyk CM, Laubenstein LJ, et al. Therapeutic trial of interferon-gamma in patients with epidemic Kaposi's sarcoma. J Biol Response Mod. 1985;4: 358-364. [PubMed] [Google Scholar]
- 63.Lane H, Sherwin S, Masur H, et al. A phase I trial of recombinant immune (gamma) interferon in patients with the acquired immunodeficiency syndrome (AIDS) [abstract]. Clin Res. 1985;33: 408A. [Google Scholar]