Figure 2. Influence of poison concentration and delay of obidoxime therapy on the reactivation of erythrocyte AChE.
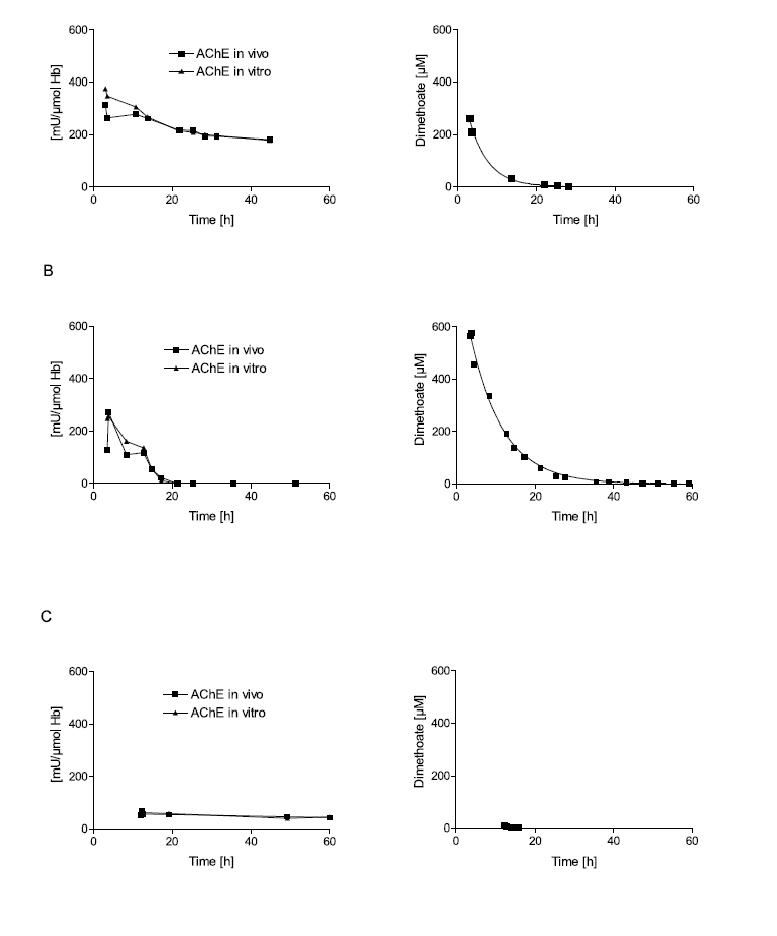
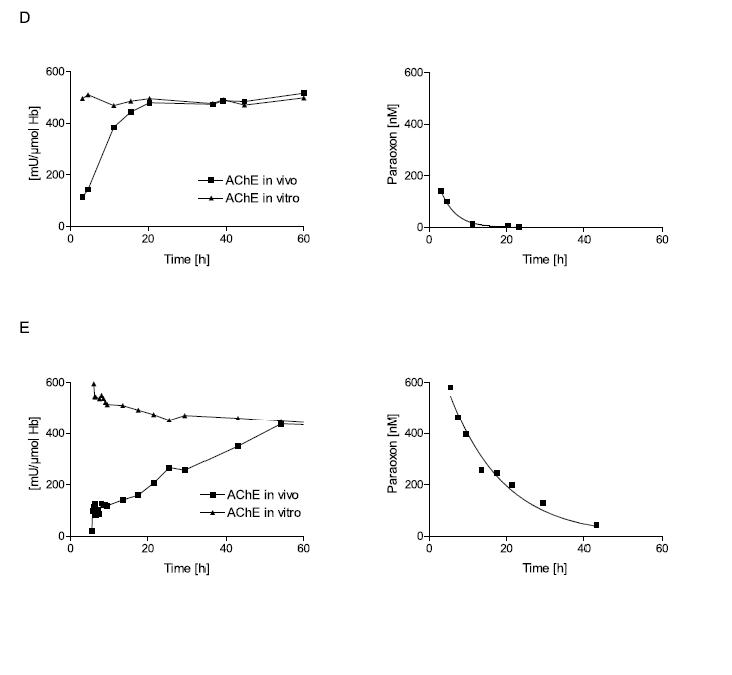
Erythrocyte AChE was determined according to Worek et al.42 and referred to the haemoglobin content of blood (mU/μmol HbFe); the normal value is about 600 mU/μmol Hb. Activity in vivo demonstrates the activity of diluted (1:100) venous blood of the patient; activity in vitro demonstrates its activity after reactivation with a supratherapeutic dose of obidoxime. This value indicates the amount of enzyme that is not aged and therefore potentially reactivateable.15 The poison concentration in plasma was determined by HPLC. All patients were treated with atropine and an IV bolus dose of 250 mg obidoxime followed by continuous infusion at 750 mg/24h for the period indicated by the time scale. This regimen produced plasma concentrations between 10 and 20 μM obidoxime. Obidoxime was started at the first time point shown in the figures; the x-axis indicates time post ingestion. Dimethoate is a dimethylphosphorylated OP, while parathion is a diethylphosphorylated OP. Patient A had ingested a moderate dose of dimethoate 3h before obidoxime administration. This therapy resulted in near complete reactivation of the unaged fraction of AChE. Since the poison concentration was low and declined rapidly, obidoxime was able to keep the enzyme activity at 1/3 of normal. Patient B had ingested a high dose of dimethoate 3.5h before obidoxime administration. While initial reactivation was observed, the enzyme activity gradually fell due to the higher and more persistent dimethoate concentration. Patient C had ingested a small dose of dimethoate about 12h before obidoxime treatment. During this period (3-4 half-lives), some 90% of the enzyme had been aged. Hence, obidoxime did not show any significant effect although the poison concentration was very low at that time. (Note that some spontaneous reactivation of the unaged AChE is always observed when the poison concentration is low.) Patient D had ingested a moderate dose of parathion 3h before administration of the obidoxime regimen. Most of the enzyme was still reactivatable and reactivation in vivo increased steadily while the paraoxon concentration in plasma declined. Patient E had ingested a high dose of parathion 5.5h before obidoxime treatment. Only a small fraction of the AChE was reactivated initially but this fraction increased during the next few days as the paraoxon concentration fell gradually. Due to the slow ageing of the diethyl-phosphorylated enzyme, reactivatability decreased only slowly. The data show that oximes are particularly effective with diethyl-OPs, provided treatment is long enough to counteract re-inhibition. With dimethyl-OPs, oximes have to be given as early as possible and are only effective at moderate poison load (Eyer et al., in preparation).
