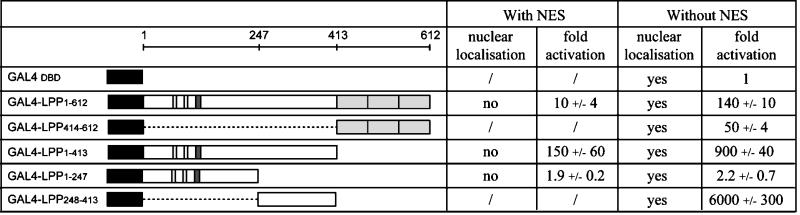
Figure 6.
Structure and transcriptional activation capacity of GAL41–147-LPP chimeric proteins. Scheme of chimeric proteins comprising the GAL4 DNA-binding domain (amino acids 1–147) and either wild-type LPP or various N-terminal and/or C-terminal deletions of LPP. The GAL4 DNA-binding domain is represented by a black box. Domain organization of LPP is as in Figure 1. The NES of LPP is shown as a dark gray box. All chimeric constructs (with NES or without NES) were cotransfected into the 293 cell line along with a GAL4-regulated luciferase reporter and an RSV-β-galactosidase internal control, and cell lysates were assayed for luciferase activity 18–24 h after transfection. Activation (fold) is relative to the control GAL4DBD (DNA-binding domain). The results are the average of three independent experiments ± SD. Subcellular localization of the different constructs was established by immunocytochemistry with the use of a GAL4 antibody.