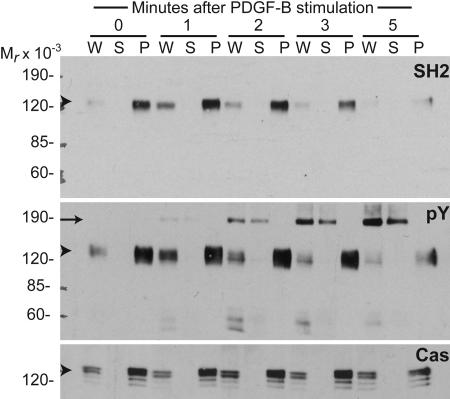
Fig. 4.
p130Cas is the major tyrosine-phosphorylated protein that binds Nck in cells stimulated with PDGF-B. Lysates obtained from NIH 3T3 cells left untreated (time 0) or stimulated with PDGF-B for various intervals were immunoprecipitated with polyclonal antibodies against p130Cas. Whole-cell lysates (W) and supernatant (S) and pellet (P) fractions were subjected to SDS/PAGE, and proteins were transferred to nitrocellulose filters. Pellet fractions represent five times more lysate than whole-cell lysate or supernatant fractions. Membranes were probed with GST fusions of the isolated SH2 domain from Nck1 (SH2) in a far-Western blot or with monoclonal anti-phosphotyrosine (pY) or anti-p130Cas (Cas) antibodies in Western blot analysis. Apparent molecular weights (Mr × 10−3) are indicated. Arrowheads indicate a band of ≈130 kDa in size corresponding to p130Cas, and the arrow indicates the PDGFβR.