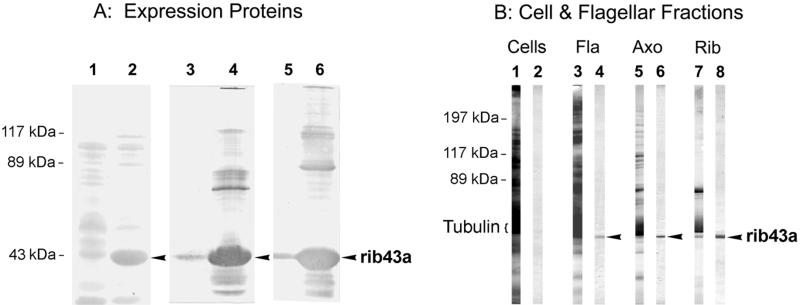
Figure 3.
Western blot analysis of antibodies raised against the native Chlamydomonas ribbon protein rib43a (anti-rib43a) and against the bacterially expressed, truncated fusion protein from pBrib43aΔN64 (anti-rib43aΔN64). (A) SDS-PAGE blots of lysates from bacteria transformed with pBrib43aΔN64, as follows: lanes 1, 3, and 5, uninduced; lanes 2, 4, and 6, induced with IPTG. Lanes 1 and 2 were stained with Ponceau S; lanes 3 and 4 were stained with anti-rib43aΔN64; and lanes 5 and 6 were stained with anti-rib43a. The IPTG-induced fusion protein rib43aΔN64 (arrowheads) is recognized both by the antibody made against it (anti-rib43aΔN64, lane 4) and by anti-rib43a (lanes 6). (B) SDS-PAGE blots of whole Chlamydomonas cells (Cells, ∼80 μg) and the sequential fractionation of flagella (Fla, 60 μg) into axonemes (Axo, 40 μg) and ribbons (Rib, 20 μg). Lanes 1, 3, 5, and 7, stained with Ponceau S; lanes 2, 4, 6, and 8, stained with affinity-purified anti-rib43aΔN64 antibodies (against the bacterially expressed fusion protein). Anti-rib43aΔN64 antibodies continue to stain the rib43a protein (arrowheads) that is retained in the purified ribbons after fractionation of flagella and axonemes. Only faint staining of rib43a could be seen in the original, freshly stained blot of whole cells; the apparent bands seen here are attributable to faint, uneven background across the nitrocellulose sheet.