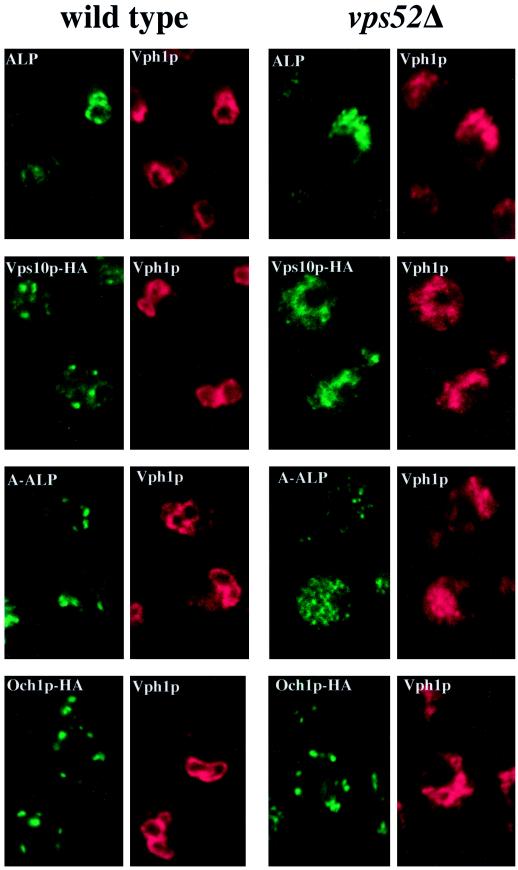
Figure 3.
Localization of membrane proteins by double-label immunofluorescence in wild-type and vps52Δ cells. (A) The vacuolar membrane protein ALP (green) colocalizes with the vacuolar ATPase subunit Vph1p (red) in wild-type (SNY36-9B) and vps52Δ (LCY222) cells containing centromere plasmid pSN92 (ALP). (B) Vps10p-HA (green) is localized to dots that are distinct from the vacuoles that label with Vph1p (red) in wild-type cells (LCY294), whereas both markers label the fragmented vacuoles of vps52Δ cells (LCY315). (C) A-ALP (green) is localized to dots that are distinct from the vacuoles that label with Vph1p (red) in wild-type cells (SNY36-9B containing pSN55), whereas in vps52Δ cells, A-ALP is found in smaller punctate structures as well as in the vacuoles of vps52Δ cells (LCY222 containing pSN55). (D) The cis-Golgi marker protein Och1p-HA (green) exhibits a similar punctate distribution in both wild-type (LCY302) and vps52Δ (LCY316) strains that is distinct from the vacuolar staining of Vph1p (red). Strains were fixed, spheroplasted, and double labeled with rabbit anti-Vph1p antiserum and a mouse mAb to either ALP (A and C) or HA (B and D) followed by fluorescently labeled secondary antibodies.