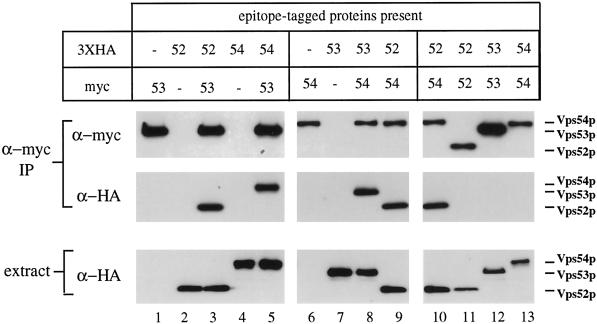
Figure 7.
Vps52p, Vps53p, and Vps54p coprecipitate as a 1:1:1 complex. Strains containing one or more epitope-tagged proteins (as indicated) were subjected to immunoprecipitation under nondenaturing conditions with affinity-purified rabbit anti-myc antibodies. Copurifying proteins were subsequently detected by Western blotting with anti-myc (top panels) or anti-HA (center panels) mouse mAbs and species-specific secondary antibodies. Equal amounts of total cell lysates, as determined by Bradford assay, were also analyzed by Western blotting to determine the relative amounts of each HA-tagged protein in the extracts (bottom panels). Blots were visualized by chemiluminescence and quantified by chemifluorescence (see MATERIALS AND METHODS). All samples were processed in parallel; lanes 9 and 10 are identical. Apparent differences in the levels of Vps52p-myc and Vps54p-myc relative to Vps53p-myc are due to context dependence of the anti-myc mAb 9E10; all three proteins are recognized equally well by the rabbit anti-myc serum (our unpublished results). The following strains were used: lane 1, LCY228; lane 2, LCY226; lane 3, LCY227; lane 4, LCY233; lane 5, LCY237; lane 6, LCY234; lane 7, LCY229; lane 8, LCY235; lane 9, LCY256; lane 10, LCY256; lane 11, LCY226 + pLC65; lane 12, LCY228 + pLC75; lane 13, LCY234 + pLC104.