Abstract
Lonidamine (LND) is a compound originally developed as an infertility drug. By capitalizing on the unique energy requirements of many solid tumors including benign prostatic hyperplasia (BPH), LND has shown efficacy as an adjunct to either radiation or chemotherapy in the treatment of several advanced solid organ malignancies such as lung, breast, head and neck, and liver metastases. It has an excellent safety profile in over 20 years of use in Italy in thousands of cancer patients. Preliminary data suggest that it is safe and effective in the treatment of lower urinary tract symptoms associated with BPH by metabolically targeting the unique dependency of the prostate on energy production by glycolysis instead of the aerobe Krebs cycle. The observed effects include a fast reduction in serum prostate-specific antigen and prostate volume, and simultaneous improvements in symptoms and urinary flow rate. The fact that prostate cancer and high-grade prostatic intraepithelial neoplasia have similar metabolic circumstances suggests that LND might also be effective in various stages of the prostate cancer carcinogenesis.
Key words: Benign prostatic hyperplasia, Lonidamine, Metabolic targeting, Krebs cycle, High-grade prostatic intraepithelial neoplasia, Cancer treatment
In many disease states the concept of targeted therapy has become very popular over the past years. Targeted therapy implies that the treatment is somehow tailored either to the underlying disease process, whether it is a pathogenic organism, a cancerous growth, or a metabolic abnormality or syndrome, or to the person suffering from the disease process. In the area of metabolic diseases, for instance, genomic approaches are excellent tools to manage genetic complexity and have been applied successfully to identify candidate target genes that might lead to the development of novel therapies for metabolic diseases.1
Another area of interest is microbiology and the treatment of pathogens. The large quantity of genomic, biochemical, and metabolic data on microbial pathogens provides information that helps to select biological problems and to identify targets, metabolic pathways, or constituent enzymes for therapeutic intervention. Developing novel antiparasitic agents concerns the regulation of oxidative stress, and the trypanothione peroxidase pathway has been targeted in this respect.2
Perhaps the most promising area of targeted therapy is oncology. The Human Genome Project, the Cancer Genome Project, and related activities will define most of the genes in the majority of common human cancers over the next 5 years. This will provide the opportunity to develop a range of drugs targeted to the precise molecular abnormalities that drive various human cancers. It also opens up the possibility of personalized therapies targeted to the molecular pathology and genomics of individual patients and their malignancies. The new molecular therapies should be more effective and have less severe side effects than cytotoxic agents.3
Metabolic Targeting
Metabolic targeting is a new approach toward small molecule therapy that takes advantage of a fundamental property of many diseases and solid tumors, namely abnormal glucose metabolism. This technology has broad potential application in the treatment of both benign and malignant tumors and holds promise to deliver significant benefits including:
prolonged remissions,
prevention of relapse,
enhanced potency of existing therapies,
increased specificity of drug delivery, and
limited side effects.
Metabolic targeting provides an opportunity to target fundamental differences in glucose metabolism between normal and diseased cells. This technology provides the opportunity to treat not only rapidly dividing tumor cells, which are also targeted by chemotherapy and radiation, but slowly dividing tumor cells within hypoxic regions. Slowly dividing tumor cells generally evade these traditional therapies and ultimately contribute to relapse.
Energy Generation Under Aerobe or Anaerobe Conditions
Cells generate energy needed for survival and growth in 2 ways: the citric acid cycle and glycolysis. The citric acid cycle, which is dependent on sufficient oxygen supply (ie, aerobe conditions), is a highly efficient process that provides the majority of cellular energy under normal conditions. Citrate synthesis and oxidation via the Krebs cycle are central to the pathways of intermediary metabolism of aerobic cells. The complete oxidation of glucose and fats is achieved through the synthesis of citrate and its oxidation via the Krebs cycle.
It is through the synthesis and oxidation of citrate that cells generate, by coupled phosphorylation, their major supply (approximately 80%) of cellular energy (ATP production). In addition, the Krebs cycle and the recycling of its intermediates provide major pathways for the biosynthesis and degradation reactions of amino acid metabolism. Synthesized citrate provides the source of acetyl-CoA required for lipogenesis.
On the other hand, a much less efficient process of glucose metabolism is glycolysis, in which energy is generated in the absence of oxygen (ie, anaerobe conditions). Glycolysis relies on large quantities of available glucose, produced by the process of gluconeogenesis. Some diseased cells rely predominantly or exclusively on glycolysis. When these cells shift energy production to glycolysis, they must increase the levels of proteins needed to transport and metabolize glucose. Metabolic targeting takes advantage of these metabolic differences to selectively target certain diseased cells.
Unique Metabolism of the Prostate Gland
One of the unique metabolic features of the human prostate gland is the fact that it accumulates and secretes extraordinarily high levels of citrate and zinc in the seminal fluid4 (Table 1). It is now evident that this function is associated specifically with the glandular epithelium of the peripheral zone. Thus secretory epithelial cells of the peripheral zone are the highly specialized zinc-accumulating, citrate-producing cells of the human prostate. In contrast, the glandular cells of the central zone do not accumulate zinc and are citrate-oxidizing cells typical of most mammalian cells. No other cells in the body exhibit these functional and metabolic capabilities that uniquely characterize the peripheral zone secretory epithelial cells.5
Table 1.
Representative Citrate and Zinc Levels in Prostate
| Tissue Analyzed | Citrate, nmol/g | Zinc, μg/g |
|---|---|---|
| Normal (mixed tissue) | 8000 | 209 |
| Normal (central zone) | 4000 | - |
| Normal (peripheral zone) | 13,000 | - |
| BPH | 8000–15,000 | 589 |
| PCa (mixed tissue) | 1000–2000 | 55 |
| PCa (malignant tissue) | 500 | - |
| Other soft tissue | 150–450 | 30 |
| Blood plasma | 90–110 | 1 |
| Prostatic fluid | 40,000–150,000 | 590 |
BPH, benign prostatic hyperplasia; PCa, prostate cancer. Reprinted with permission from Costello and Franklin.4
Another unique aspect of prostate metabolism is the dependence on glycolysis for energy production due to an enzymatic block in the oxidative (aerobe) phosphorylation or Krebs cycle. The inhibition of the Krebs cycle, and the necessity of the prostate to use the less efficient process of anaerobe glycolysis, has been extensively reviewed and substantiated.4 This dependence results from a zinc-mediated enzymatic block in the citric acid cycle that mediates an essential function of the prostate gland—the secretion of extraordinarily high levels of citrate and zinc.4 In this context, it is interesting to note that, like many other solid organ tumors, prostate cancer is distinctly hypoxic compared to normal prostate tissue (see Table 2).
Table 2.
Most Solid Tumor Including Prostate Cancer Are Hypoxic: Median pO2 Shown in mm Hg
| Median Tumor pO2* | Median Normal pO2* | |
|---|---|---|
| Tumor Type | (Number of Patients) | (Number of Patients) |
| Glioblastoma | 4.9 (10) | ND |
| 5.6 (14) | ND | |
| Head and neck carcinoma | 12.2 (30) | 40.0 (14) |
| 14.7 (23) | 43.8 (30) | |
| 14.6 (65) | 51.2 (65) | |
| Lung cancer | 7.5 (17) | 38.5 (17) |
| Breast cancer | 10.0 (15) | ND |
| Pancreatic cancer | 2.7 (7) | 51.6 (7) |
| Cervical cancer | 5.0 (8) | 51 (8) |
| 5.0 (74) | ND | |
| 3(86) | ND | |
| Prostate cancer | 2.4 (59) | 30.0 (59) |
| Soft-tissue sarcoma | 6.2 (34) | ND |
| 18 (22) | ND |
pO2 measured in mm Hg. Measurements were made using a commercially available oxygen electrode (the “Eppendorf” electrode). The values shown are the median of the median values for each patient. ND, not determined; pO2, oxygen partial pressure. Adapted from Brown and Wilson27
Lonidamine
Lonidamine (LND), a derivate of indazole-3-carboxylic acid, is thought to disrupt energy metabolism by interfering with glycolysis. It inhibits ADP-and uncoupler-stimulated respiration on various NAD- and FAD-linked substrates,6 inhibits oxygen consumption and hexokinase activity in Ehrlich ascites tumor cells,7,8 and selectively induces apoptosis in a citrate-producing prostate cell line.9 LND also induces a reduction in lactate levels in tumor cells.8 Originally developed as a nonhormonal male contraceptive, LND's effect is believed to be mediated by impairment of Sertoli/sperm interaction, and it induces a reversible reduction in sperm count with no effect on serum testosterone.
Nonclinical Studies With LND
LND has been studied for potential mutagenicity in a comprehensive battery of tests. In assays for the induction of gene mutations in prokaryotes (Ames test) and eukaryotes (induction of HPRT mutations in Chinese hamster ovary [CHO] cells), negative results were obtained. There was no evidence of the induction of chromosomal damage in cultured mammalian cells in vitro. No mutagenic activity was observed in tests for chromosomal damage in vivo, in somatic cells (micronucleus test), or in germinal cells (dominant lethal test). These negative results are consistent with observations indicating that LND affects cellular energy processes rather than the mechanisms of cell division.
Plasma LND concentration and toxicity were investigated in dogs receiving LND orally twice daily for 30 days or a single intravenous dose up to 1200 mg/m2. Physical or laboratory signs of toxicity were not observed in dogs receiving oral LND. Therefore, this dose and route appear to be viable for in vivo studies.10,11
Clinical Studies With LND
The pharmacokinetics of LND has been studied in cancer subjects after single and long-term oral administrations. These LND studies in humans showed a wide variation of the plasma concentration-time profiles following a single oral dose. An additional study was performed involving 12 subjects with non-small-cell malignancies of the lungs. Results indicate that steady state was reached after 2 dosing intervals of 12 hours, and no changes in liver metabolism or age-dependent pharmacokinetics were present after 4 days of multiple dose treatment.12
Lonidamine is devoid of conventional side effects induced by antiproliferative agents (ie, myelosuppression, stomatitis, cystitis, alopecia, renal, hepatic, and cardiac toxicity). No serious or life-threatening adverse reactions have been recorded even over long-term treatment periods. Given as a single agent (in daily doses ranging between 300 and 900 mg) LND induces the following mild side effects: myalgia, testicular pain, asthenia, ototoxicity, nausea and vomiting, gastric pain, and drowsiness. Hyperesthesia and photophobia also have been reported in early studies but not reported in randomized studies. In combination with radiotherapy (in oral daily doses ranging between 300 and 450 mg) LND was well tolerated, without any reported evidence of additional toxicity. When LND was associated with cytotoxic agents no enhanced toxicity was observed, except for reversible myalgias. In particular, myelosuppression and other conventional nonhematological adverse reactions were never greater than would be expected with chemotherapy alone.
The data collected from the large series of cancer subjects treated with this new agent show that LND is a safe drug whether used alone or in combination with other effective anticancer treatments. Approved in Italy for oncology indications, LND has been studied extensively in humans for more than 20 years, and generally shows only mild, temporary side effects.11 The reported therapeutic efficacy and the peculiar toxicity profile make LND an interesting new drug for future clinical trials.
LND in the Treatment of BPH
Based on the clinical safety history of LND, its potential novel mechanism of action, and preclinical data, a study was initiated to investigate the effects of once-daily oral doses of LND on prostate volume, prostate specific antigen (PSA), urine flow, and International Prostate Symptom Score (IPSS) in men with symptomatic benign prostatic hyperplasia (BPH).
LND represents a novel therapeutic approach to BPH, exploiting the unique metabolic environment of the human prostate. Because of its high zinc content, the prostate depends on glycolysis for energy production. Inhibition of the first hexokinase-dependent critical step in the glycolysis by LND would theoretically render the prostate without any of the 2 common sources of energy production: the Krebs cycle hindered by the zinc levels, and the glycolysis rendered inactive by hexokinase enzyme inhibition.
In animal models, a single oral dose of an LND analogue has been shown to reduce the size of the rat prostate by 24%, and multiple dosing results in even greater reduction. This reduction, occurring at doses that cause no other clinical effect on the animals, has been reproduced with different regimens by different investigators.13 Although testicular weight also decreased in rats, studies in men demonstrated no effect on serum testosterone.
One single-arm open-label phase II study of LND in subjects with BPH is currently in the follow-up phase (TH-CR-201). LND (150 mg) was administered orally every day to 30 subjects for 28 days. No significant adverse events were attributed to the study drug. Significant responses (IPSS, Qmax [maximum flow rate], residual urine and prostate volume) were seen in subjects at day 14 and day 28 compared to baseline. The IPSS decreased from a mean of 19.5 to 12.2 points, Qmax increased from a mean of 9.4 to 12.6 mL/s, and mean prostate volume decreased from 55.4 to 49.6 mL by transrectal ultrasound of the prostate (TRUS). Simultaneously, the serum PSA dropped from 3.6 to 2.8 ng/mL. LND was well tolerated with no treatment-related side effects reported (one subject experienced a nondrug related hematuria). Study subjects are currently in extended follow-up to monitor intermediate term results.14
Future Development of LND for LUTS and BPH
Several phase II/III clinical studies are planned to start in both Europe and the United States in late 2005. The primary objective will be to evaluate the efficacy as measured by IPSS of LND (50 mg and 150 mg) compared to placebo in subjects with symptomatic BPH. The secondary objectives will be to evaluate the efficacy (Qmax on uroflowmetry, prostate volume, and PSA) and safety of LND (50 mg and 150 mg) compared to placebo.
Trial Design
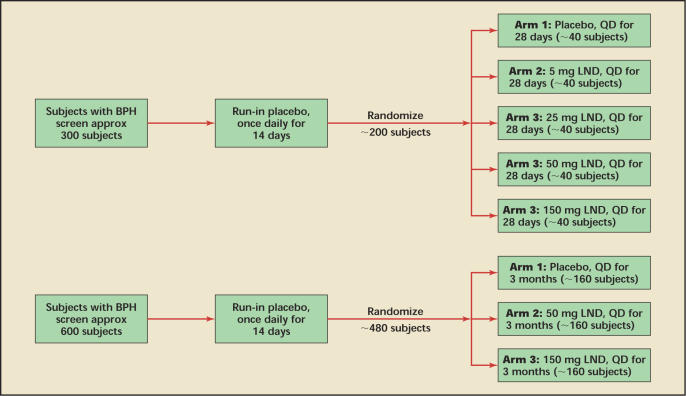
In the United States, the emphasis will be on dose ranging and pharmacokinetics. Approximately 200 subjects will be treated with 5, 25, 50, and 150 mg LND, respectively, versus placebo over 1 month. In Europe, 480 subjects will be randomized to placebo versus LND 50 mg versus LND 150 mg over 3 months. The similar entry criteria and the fact that both studies include a 50 mg and 150 mg dose will help determine both appropriate treatment and dosage duration while providing substantial safety data on the likely daily dosage.
Subjects will be assessed at screening prior to placebo run-in treatment, at baseline (randomization following placebo run-in, day 14), and following double-blind treatment for IPSS, Qmax by uroflowmetry, prostate volume by TRUS, PSA, adverse events, hematology, and serum chemistry. Subjects will return for follow-up evaluations at 3 months and at 6 months post dosing (Figure 1).
Figure 1.
Proposed scheme for the US (top) and the European (bottom) LUTS and BPH trials. Both trials include a follow-up period of a minimum of 6 months. LUTS, lower urinary tract symptoms; BPH, benign prostatic hyperplasia; LND, lonidamine; QD, every day. Source: Threshold Pharmaceuticals, data on file.
Study Population
Eligibility Criteria
Qualified subjects must meet the following criteria: (1) prostate volume measured by TRUS > 30 cc within the last month, (2) Qmax < 15 mL/s when measured by uroflowmetry within the last 2 days, (3) IPSS > 13 within the last 2 days, (4) PSA > 1.0 ng/mL within the last month (a second measurement must be taken at baseline but results are not required for eligibility), (5) at least 12 doses must be taken during the run-in treatment period, and (6) able to comply with the prescribed treatment protocol and evaluations.
Inclusion Criteria
Eligible subjects will have to meet the following criteria: (1) capable of understanding the purpose and risks of the study and signing a statement of informed consent, (2) male 50 to 80 years, (3) presence of LUTS for at least 3 months, (4) prostate volume measured by TRUS > 30 cc, (5) Qmax < 15 mL/s when measured by uroflowmetry, (6) IPSS > 13, (7) PSA > 1.0 ng/mL, and (8) able to comply with the prescribed treatment protocol and evaluations.
Exclusion Criteria
Subjects will be ineligible for this study based on any one of the following criteria: (1) prior treatment for BPH other than α-blockers (α-blockers are not allowed during the study and for 14 days prior to screening), (2) prior surgery of the prostate (except biopsies), (3) current or past evidence of malignant disease of the prostate (subjects with PSA > 4 ng/mL should undergo ultrasound-guided prostate biopsy to rule out malignant prostate disease), (4) active cardiac, renal, or hepatic disease as evidenced by creatinine ≥ 1.8 mg/dL, ALT or AST ≥ 2.5x the upper limit of normal at screen, history of myocardial infarction, congestive heart failure or unstable cardiac arrhythmias within 6 months prior to study entry, (5) uncontrolled diabetes mellitus (fasting blood glucose > 200 mg/dL), (6) concurrent participation or participation in an investigational drug study within the past 30 days, or (7) concomitant disease or condition that could interfere with the conduct of the study or would, in the investigator's opinion, pose an unacceptable risk to the subject in this study.
Statistical Determination of Sample Size
Sample size calculations are based on the primary efficacy variable, namely changes in the IPSS. A decrease or improvement of −2 points for placebo versus −5 points for LND (ie, a net benefit of 3 points) is assumed with a standard deviation of 6 points derived from a review of relevant literature. A total decrease of −3 points in the IPSS has been shown by Barry and colleagues15 to be clinically significant for men with LUTS and BPH. It is estimated that both trials will be finalized and submitted to the respective institutional review boards with the first patient enrolled in 2005.
Oncologic Indications for LND
Lonidamine has been experimentally shown to potentiate the cytotoxic effects of anthracyclines in human breast cancer cell lines and cisplatin activity in both platinum-sensitive and platinum-resistant human ovarian carcinoma cell lines.16,17 Because the specific action mechanism and side effects are not overlapping with those of standard antineoplastic agents, the combination of LND with standard chemotherapy has been widely investigated for the treatment of solid tumors.12,18–23 In addition, LND's enhancement of radiotherapy activity has been evaluated in head and neck cancer, localized non-small-cell lung cancer, brain cancer, and brain metastases.24,25
When used as an adjunct to conventional chemotherapy, LND has shown improved survival in advanced lung cancer18 and advanced breast cancer, and has shown reduction in the size of liver metastases.26 Table 3 (on the previous page) provides an overview of the clinical trial results with LND in advanced solid organ cancer26,27 and Table 4 provides an overview of the limited toxicity seen in those trials.18,20,24,26,28–37
Table 3 .
Clinical Trials With Lonidamine in Advanced Cancer
| No. of | ||||
|---|---|---|---|---|
| Patients | Patient Population | Study Design | Dose | Total Dose |
| 31 | Various solid tumors | Phase 1 | 180–520 mg/mg2/day | At least 28 days |
| (∼306–884 mg/day*) | ||||
| 12 | Various metastatic cancers | Phase 2, single-arm | 270 mg/mg2/day (∼459 mg/day) | 2200–76,800 mg |
| 30 | Various solid tumors | Phase 2, single-arm | Up to 450 mg/m2 (∼765 mg/day) | Median 180 days |
| 64/60/60 | Lung cancer | Randomized | 600 mg/day | 58 days |
| 141/163 | Lung cancer | Randomized | 450 mg/day | Median 10 months |
| 30/32/33 | Elderly lung cancer | Randomized | 450 mg/day | At least 8 weeks |
| 104/103 | Breast cancer | Randomized | 600 mg/day (in combination | Median 126 days |
| with epirubicin) | ||||
| 65/69 | Breast cancer | Randomized | 600 mg/day (in combination | Average 60 days |
| with doxorubicin) | ||||
| 57/55 | Breast cancer | Randomized | 600 mg/day (in combination | Median 9 months |
| with chemotherapy) | ||||
| 80/78 | Lung cancer | Randomized | 450 mg/day | Approximately 7 months |
| 49/48 | Head and neck cancer | Randomized | 450 mg/day | 8 months |
| 152/158 | Lung cancer | Randomized | 265 mg/mg2/day (∼450 mg/day) | 230 days |
| 160/148 | Breast cancer | Randomized | 600 mg/day | Until progression about 9 months |
| 128/126 | Breast cancer | Randomized | 600 mg/day | Until progression about 9 months |
Prostate Cancer and Precancerous Lesions
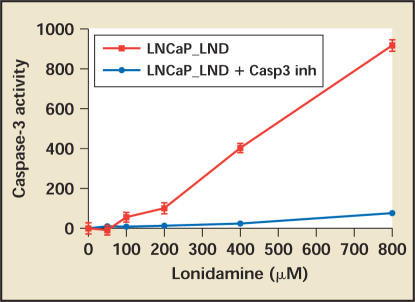
LND has demonstrated activity in vitro against hormone-dependent prostate cancer cell lines, induces caspase-3-mediated apoptosis (Figure2), and shows a statistically significant reduction in epithelial tumors in rats given long-term treatment. High-grade prostatic intraepithelial neoplasia (HGPIN) and low-grade prostate cancer have the same biology as normal/BPH epithelium, namely a block in the citric acid cycle, and theoretically should be susceptible to the same metabolic targeting outlined above for the treatment of LUTS and BPH. Future developments of LND in urologic oncology might therefore include a variety of trials in different stages of prostate cancer.
Figure 2.
LND induces apoptosis in LNCaP hormone-sensitive prostate cancer cells in vitro. LND, lonidamine; LNCaP, human cancer prostate cells; Casp3inh, caspase-3 inhibitor. Source: Threshold Pharmaceuticals, data on file.
High Grade Prostatic Intraepithelial Neoplasia
HGPIN is a recognized premalignant lesion. Several trials are underway targeting HGPIN patients to prevent progression to overt prostate cancer. One such trial is associated with the SELECT prostate cancer prevention trial and represents a substudy, in which patients with diagnosed HGPIN are given selenium and/or vitamin E, similar to the design of the overall SELECT trial.38 Other trials explore 5α-reductase inhibitors and other compounds for the same purpose. It is conceivable that LND might have activity in this indication.
A trial could be designed enrolling patients diagnosed with HGPIN on TRUS-guided biopsy to be randomized to LND versus placebo and subjected to a repeat biopsy at a specified time point (eg, 6 months). The primary efficacy parameter would be the rate of detection of prostate cancer in subsequent biopsies in the LND versus placebo groups. A reduction of 20% to 30% might be considered clinically significant.
Established Prostate Cancer
In established prostate cancer LND might be explored in several different settings:
as an adjunct to radiation therapy, either neoadjuvant or adjuvant
as an adjunct to surgery in a neoadjuvant setting
as an adjuvant treatment following radical surgery in patients with high risk for biochemical or metastatic recurrence
as an adjunct to the standard hormonal therapy for metastatic prostate cancer with the primary endpoint being improved survival and the intermediate endpoint being an extension of the hormonally responsive state
as an adjunct in the treatment of androgen insensitive prostate cancer (AIPC)
Admittedly, little if anything is known about the metabolic features of established prostate cancer stratified by Gleason grade, metastatic prostate cancer, or even AIPC. Thus, considerable preliminary work might need to be done prior to embarking on such studies. On the other hand, the benign adverse event profile of LND and the desperate situation of patients with AIPC might make a hypothesisgenerating trial reasonably attractive.
Conclusions
Developed as an infertility drug, LND capitalizes on the unique energy requirements of many solid tumors including BPH, and is effective as an adjunct to either radiation or chemotherapy in the treatment of several advanced solid organ malignancies. These include lung, breast, head and neck, and liver metastases. It has an excellent safety profile based on over 20 years of use in Italy on thousands of cancer patients.
Preliminary data suggest that it is safe and effective in the treatment of LUTS associated with BPH by metabolically targeting the unique dependency of the prostate on energy production by glycolysis instead of the aerobe Krebs cycle. The observed effects include a fast reduction in serum PSA and prostate volume, and simultaneous improvements in symptoms and urinary flow rate. The fact that prostate cancer and HGPIN have similar metabolic circumstances suggests that LND might also be effective in various stages of the prostate cancer carcinogenesis.
Main Points.
Originally developed as a nonhormonal male contraceptive, lonidamine (LND), a derivate of indazole-3-carboxylic acid, is thought to disrupt energy metabolism by interfering with glycolysis. When LND was tested for potential mutagenicity in nonclinical studies, negative results were obtained, indicating that it affects cellular energy processes rather than the mechanisms of cell division.
Approved in Italy for oncology indications, LND has been studied extensively in humans for more than 20 years, and is shown to be a safe drug whether used alone or in combination with other anticancer treatments. It generally induces only mild, temporary side effects.
LND represents a novel therapeutic approach to benign prostatic hyperplasia (BPH). In one single-arm open-label phase II study of BPH subjects currently in the follow-up phase, significant responses were seen in subjects at day 14 and day 28 compared to baseline. The IPSS decreased from a mean of 19.5 to 12.2 points, maximum flow rates increased from a mean of 9.2 to 12.5 mL/s, and mean prostate volume decreased from 55.4 to 49.6 mL by transrectal ultrasound. Simultaneously, the serum PSA dropped from 3.6 to 2.8 ng/ml. Several phase II/III clinical studies in both the United States and Europe will be initiated in late 2005.
The combination of LND with standard chemotherapy has been widely investigated for the treatment of solid tumors and has shown improved survival in advanced lung cancer and advanced breast cancer, and reduction in the size of liver metastates. In addition, its use with radiotherapy has been evaluated in head and neck cancer, localized non-small-cell lung cancer, brain cancer, and brain metastases.
High-grade prostatic intraepithelial neoplasia and low-grade prostate cancer have the same biology as normal/BPH epithelium, and, theoretically, LND should be effective in various stages of the prostate cancer carcinogenesis.
Table 4.
Toxicity by WHO Criteria in Cancer Trials With LND
| WHO Toxicity Grades (%)* |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | P | |
| Nausea/vomiting | NS | |||||
| EPI | 16 | 24 | 43 | 17 | 0 | |
| EPI + LND | 19 | 21 | 46 | 14 | 0 | |
| Stomatitis | NS | |||||
| EPI | 65 | 18 | 10 | 7 | 0 | |
| EPI + LND | 67 | 18 | 13 | 1 | 1 | |
| Diarrhea | NS | |||||
| EPI | 90 | 3 | 6 | 1 | 0 | |
| EPI + LND | 92 | 2 | 5 | 1 | 0 | |
| Liver | NS | |||||
| EPI | 97 | 1 | 2 | 0 | 0 | |
| EPI + LND | 96 | 2 | 0 | 2 | 0 | |
| Neurotoxicity | NS | |||||
| EPI | 99 | 0 | 0 | 1 | 0 | |
| EPI + LND | 99 | 1 | 0 | 0 | 0 | |
| Fever | NS | |||||
| EPI | 86 | 6 | 8 | 0 | 0 | |
| EPI + LND | 81 | 5 | 11 | 2 | 1 | |
| Cardiac | NS | |||||
| EPI | 97 | 3 | 0 | 0 | 0 | |
| EPI + LND | 98 | 2 | 0 | 0 | 0 | |
| Alopecia | NS | |||||
| EPI | 6 | 5 | 31 | 58 | 0 | |
| EPI + LND | 9 | 4 | 32 | 53 | 2 | |
| Myalgia | <.001 | |||||
| EPI | 86 | 10 | 4 | 0 | 0 | |
| EPI + LND | 58 | 19 | 13 | 8 | 2 | |
| Asthenia | NS | |||||
| EPI | 55 | 25 | 15 | 4 | 1 | |
| EPI + LND | 53 | 24 | 17 | 6 | 0 | |
| WBC | NS | |||||
| EPI | 55 | 25 | 15 | 4 | 1 | |
| EPI + LND | 54 | 23 | 16 | 7 | 0 | |
| Nadir WBC† | NS | |||||
| EPI | 3 | 9 | 33 | 49 | 6 | |
| EPI + LND | 13 | 13 | 24 | 35 | 15 | |
| Platelets | NS | |||||
| EPI | 100 | 0 | 0 | 0 | 0 | |
| EPI + LND | 97 | 1 | 2 | 0 | 0 | |
| Nadir Platelets† | NS | |||||
| EPI | 76 | 12 | 8 | 4 | 0 | |
| EPI + LND | 74 | 11 | 7 | 8 | 0 | |
| Hemoglobin | NS | |||||
| EPI | 43 | 40 | 13 | 4 | 0 | |
| EPI + LND | 42 | 40 | 14 | 4 | 0 | |
For each patient, the most severe instance of toxicity is taken into account.
Hematologic toxicity at nadir was recorded in 67 EPI patients and in 62 EPI + LND patients. WHO, World Health Organization; NS, not significant; EPI, epirubicin; LND, lonidamine; WBC, white blood cell count. Adapted with permission from Dogliotti et al26
References
- 1.Dohrmann CE. Target discovery in metabolic disease. Drug Discov Today. 2004;9:785–794. doi: 10.1016/S1359-6446(04)03223-4. [DOI] [PubMed] [Google Scholar]
- 2.Hunter WN, Alphey MS, Bond CS, Schuttelkopf AW. Targeting metabolic pathways in microbial pathogens: oxidative stress and anti-folate drug resistance in trypanosomatids. Biochem Soc Trans. 2003;31(pt 3):607–610. doi: 10.1042/bst0310607. [DOI] [PubMed] [Google Scholar]
- 3.Workman P. The opportunities and challenges of personalized genome-based molecular therapies for cancer: targets, technologies, and molecular chaperones. Cancer Chemother Pharmacol. 2003;52(suppl 1):S45–S56. doi: 10.1007/s00280-003-0593-0. [DOI] [PubMed] [Google Scholar]
- 4.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron ESG, Huggins C. The metabolism of isolated prostatic tissue. J Urol. 1944;51:630–634. [Google Scholar]
- 6.Granger DL, Lehninger AL. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982;95:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floridi A, Lehninger AL. Action of the antitumor and antispermatogenic agent lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Arch Biochem Biophys. 1983;226:73–83. doi: 10.1016/0003-9861(83)90272-2. [DOI] [PubMed] [Google Scholar]
- 8.Floridi A, Paggi MG, D'Atri S, et al. Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res. 1981;41:4661–4666. [PubMed] [Google Scholar]
- 9.Data on file, Threshold Pharmaceuticals.
- 10.Price GS, Page RL, Riviere JE, et al. Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother Pharmacol. 1996;38:129–135. doi: 10.1007/s002800050460. [DOI] [PubMed] [Google Scholar]
- 11.Robustelli Dela Cuna G, Pedrazzoli P. Toxicity and clinical tolerance of lonidamine. Semin Oncol. 1991;18(2) suppl 4:18–22. [PubMed] [Google Scholar]
- 12.Gatzemeier U, Toomes H, Picollo R, et al. Single- and multiple-dose pharmacokinetics of lonidamine in patients suffering from non-small-cell lung cancer. Arzneimittelforschung. 1991;41:436–439. [PubMed] [Google Scholar]
- 13.Heywood R, James RW, Barcellona PS, et al. Toxicological studies on 1-substituted indazole-3-carboxylic acids. Chemotherapy. 1981;27(suppl 2):91–97. doi: 10.1159/000238049. [DOI] [PubMed] [Google Scholar]
- 14.Ditonno P, Battaglia M, Selvaggio O, et al. Lonidamine treatment significantly reduces prostate volume and improves urine flow in symptomatic benign prostatic hyperplasia: results of a phase II open-label study. Prostate Cancer Prostatic Dis. Submitted for publication. [Google Scholar]
- 15.Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–1774. doi: 10.1016/s0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 16.Savini S, Zoli W, Nanni O, et al. In vitro potentiation by lonidamine of the cytotoxic effect of adriamycin on primary and established breast cancer cell lines. Breast Cancer Res Treat. 1992;24:27–34. doi: 10.1007/BF01832355. [DOI] [PubMed] [Google Scholar]
- 17.Orlandi L, Zaffaroni N, Gornati D, et al. Potentiation of cisplatin cytotoxicity by lonidamine in primary cultures of human ovarian cancer. Anticancer Res. 1994;14:1161–1164. [PubMed] [Google Scholar]
- 18.Ianniello GP, De Cataldis G, Comella P, et al. Cisplatin, epirubicin, and vindesine with or without lonidamine in the treatment of inoperable non-small-cell lung carcinoma. Cancer. 1996;78:63–69. doi: 10.1002/(SICI)1097-0142(19960701)78:1<63::AID-CNCR11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Portalone L, Lombardi A, Antilli A, et al. Treatment of inoperable non-small cell lung carcinoma stage IIIB and IV with cisplatin, epidoxorubicin, vindesine and lonidamine: a phase II study. Tumori. 1999;85:239–242. doi: 10.1177/030089169908500405. [DOI] [PubMed] [Google Scholar]
- 20.Calabresi F, Di Lauro L, Marolla P, et al. Fluorouracil, doxorubicin, and cyclophosphamide versus fluorouracil, doxorubicin, and cyclophosphamide plus lonidamine for the treatment of advanced breast cancer: A multicentric randomized clinical study. Semin Oncol. 1991;18:66–72. [PubMed] [Google Scholar]
- 21.Berruti A, Bitossi F, Gorzegno G, et al. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of a phase III study with a factorial design. J Clin Oncol. 2002;20:4150–4159. doi: 10.1200/JCO.2002.08.012. [DOI] [PubMed] [Google Scholar]
- 22.De Lena M, Lorusso V, Bottalico C, et al. Revertant and potentiating activity of lonidamine in patients with ovarian cancer previously treated with platinum. J Clin Oncol. 1997;15:3208–3213. doi: 10.1200/JCO.1997.15.10.3208. [DOI] [PubMed] [Google Scholar]
- 23.De Lena M, Lorusso V, Latorre A, et al. Paclitaxel, cisplatin, and lonidamine in advanced ovarian cancer. A phase II study. Eur J Cancer. 2001;37:364–368. doi: 10.1016/s0959-8049(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 24.Cionini L. Radiotherapy plus lonidamine in non-small lung cancer. Semin Oncol. 1991;18:49–52. [PubMed] [Google Scholar]
- 25.Scarantino CW, Newton RE, Pagiarino DA, DeGregoria M. Lonidamine in head and neck cancer: an overview. Semin Oncol. 1991;18:28–32. [PubMed] [Google Scholar]
- 26.Dogliotti L, Berruti A, Buniva T, et al. Lonidamine significantly increases the activity of epirubicin in patients with advanced breast cancer: results from a multicenter prospective randomized trial. J Clin Oncol. 1996;14:1165–1172. doi: 10.1200/JCO.1996.14.4.1165. [DOI] [PubMed] [Google Scholar]
- 27.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 28.Young CW, Currie VE, Kim JH, et al. Phase I and clinical pharmacologic evaluation of lonidamine in patients with advanced cancer. Oncology. 1984;41(suppl 1):60–65. doi: 10.1159/000225888. [DOI] [PubMed] [Google Scholar]
- 29.Band PR, Deschamps M, Besner JG, et al. Phase II study of lonidamine in cancer patients. Oncology. 1984;41(suppl 1):66–68. doi: 10.1159/000225889. [DOI] [PubMed] [Google Scholar]
- 30.Band PR, Maroun J, Pritchard K, et al. Phase II study of lonidamine in patients with metastatic breast cancer: a National Cancer Institute of Canada Clinical Trials Group Study. Cancer Treat Rep. 1986;70:1305–1310. [PubMed] [Google Scholar]
- 31.Gatzemeier U, Cavalli F, Haussinger AK, et al. Phase III trial with and without lonidamine in non-small cell lung cancer. Semin Oncol. 1991;18(suppl 4):42–48. [PubMed] [Google Scholar]
- 32.De Marinis F, Rinaldi M, Ardizzoni A, et al. The role of vindesine and lonidamine in the treatment of elderly patients with advanced non-small cell lung cancer: a phase III randomized FONICAP trial. Italian Lung Cancer Task Force. Tumori. 1999;85:177–182. doi: 10.1177/030089169908500306. [DOI] [PubMed] [Google Scholar]
- 33.Amadori D, Frassinati GL, DeMatteis A, et al. Modulating effect of lonidamine on response to doxorubicin in metastatic breast cancer patients: results from a multicenter prospective randomized trial. Breast Cancer Res Treat. 1998;49:209–217. doi: 10.1023/a:1006063412726. [DOI] [PubMed] [Google Scholar]
- 34.Gallo-Curcio C, Verturo I, Rinaldi M, et al. Chemotherapy/radiation therapy plus/minus lonidamine in the treatment of non-small cell lung cancer (limited disease): preliminary results. Semin Oncol. 1988;15(6) suppl 7:26–31. [PubMed] [Google Scholar]
- 35.Magno L, Terraneo F, Bertoni F, et al. Double-blind randomized study of lonidamine and radiotherapy in head and neck cancer. Int J Radiat Oncol Biol Phys. 1994;29:45–55. doi: 10.1016/0360-3016(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 36.Scarantino CW, McCunniff AJ, Evans G, et al. A prospective randomized comparison of radiation therapy plus lonidamine versus radiation therapy plus placebo as initial treatment of clinically localized but nonresectable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1994;29:999–1004. doi: 10.1016/0360-3016(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 37.Pacini P, Rinaldini M, Algeri R, et al. FEC (5-fluorouracil, epidoxorubicin and cyclophosphamide) versus EM (epidoxorubicin and mitomycin-C) with or without lonidamine as first-line treatment for advanced breast cancer. A multicentric randomized study. Eur J Cancer. 2000;36:966–975. doi: 10.1016/s0959-8049(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 38.Klein E. Selenium and vitamin e cancer prevention trial. Ann N Y Acad Sci. 2004;1031:234–241. doi: 10.1196/annals.1331.023. [DOI] [PubMed] [Google Scholar]