Figure 1.
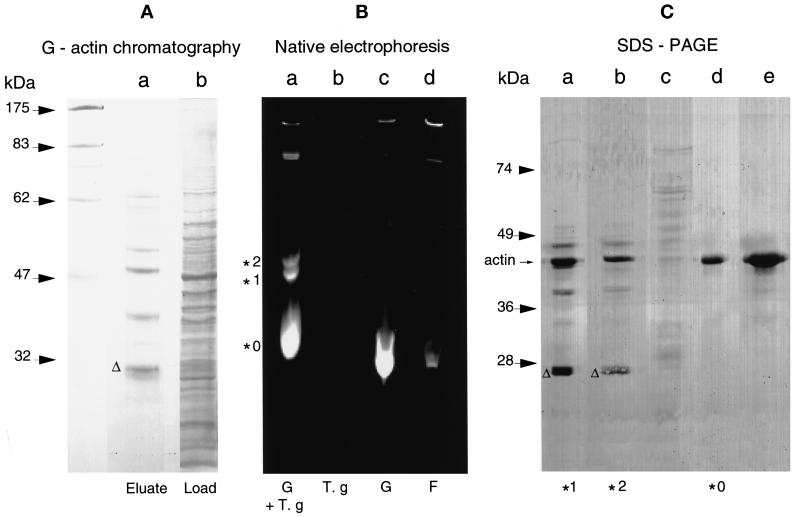
The T. gondii 27-kDa protein binds to G-actin. (A) G-actin chromatography. A 100,000 × g T. gondii cytosolic extract was subjected to affinity chromatography over a rabbit muscle G-actin column. Proteins bound to G-actin were eluted by 3 mM MgCl2 and 1 mM ATP. These proteins were further separated by SDS-PAGE on a 12% gel, transferred to a nitrocellulose membrane, and visualized with red Ponceau S stain. Lane b, cytosolic fraction corresponding to 5 × 107 T. gondii tachyzoites; lane a, eluate. The polypeptide migrating with a molecular mass of ∼27 kDa is marked with a triangle. (B) Native gel electrophoresis. A 100,000 × g parasite cytosolic extract (T.g) was incubated with IAEDANS-labeled G-actin (G), and the protein complexes were separated by native gel electrophoresis. The bands were visualized under a UV lamp (λ = 312 nm). Lane a, three bands were detected and marked, respectively, *0, *1, and *2; lane b, parasite cytosolic extract without fluorescent actin; lane c, fluorescent G-actin; lane d, fluorescent G-actin incubated under polymerization conditions to reach steady state before native gel electrophoresis. (C) Ponceau S staining on blot. Slices of the native gel (see B) containing either complex *0, *1, or *2 or from the adjacent lanes from positions corresponding to the migration of *0, *1, or *2 were boiled in SDS-PAGE sample buffer and analyzed on a 12% gel. The separated proteins were subsequently transferred onto a nitrocellulose membrane and stained with Ponceau S. Lanes a and b, material from bands *1 and *2, respectively. Apart from actin, three other similar bands were detectable in the complexes *1 and *2, including a band of ∼27 kDa (triangle); lane c, sample extracted from a slice cut from lane b on the native gel. The slice corresponded to polypeptides comigrating with *1; lane d, material from the band *0 from the native gel; lane e, material containing G-actin from lane c of the native gel.