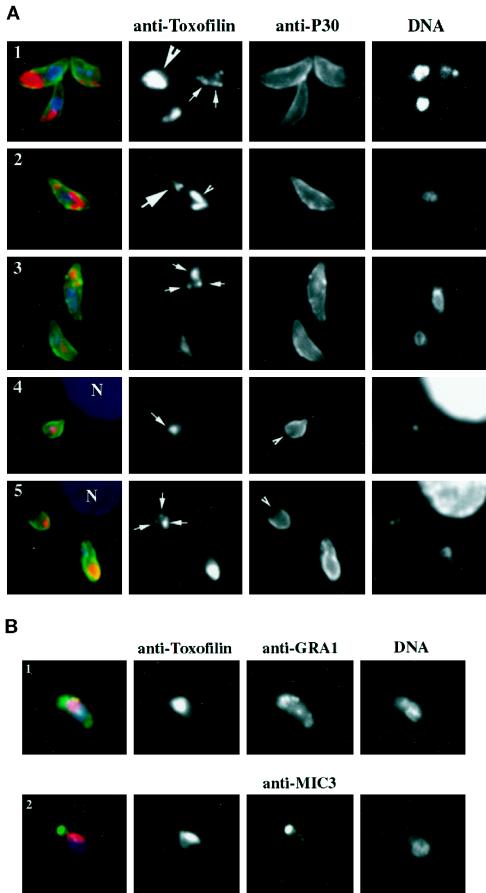
Figure 6.
Toxofilin localization during tachyzoite motility and host cell entry is dynamic. (A, 1–3) Tachyzoites were allowed to glide on glass slides before being fixed and stained for the major surface protein P30 with mouse monoclonal anti-P30 followed by a secondary anti-mouse antibody conjugated to Alexa-488. Cells were then permeabilized and stained for Toxofilin using the anti-Toxofilin antibody followed by an anti-rabbit antibody conjugated to Alexa-568. Nuclei were stained by DAPI (see MATERIALS AND METHODS). In green, the surface of the parasite; in red, Toxofilin distribution; in blue, nuclei. (A, 4 and 5) Tachyzoites were incubated for 10 min with HFF cells plated on glass coverslips. Coverslips were washed in PBS and processed for immunofluorescence (see MATERIALS AND METHODS) either immediately or after further incubation for 10 or 20 h (37°C, 5% CO2). Extracellular tachyzoites were stained with the anti-P30 followed by the anti-mouse antibody conjugated to Alexa-488. The cells were subsequenty permeabilized and stained for Toxofilin followed by an anti-rabbit antibody conjugated to Alexa 568. Nuclei were stained by DAPI. Extracellular parasites were detected by the green fluorescent staining on their surface, whereas tachyzoites in the process of entering the host cell present an incomplete staining of their surface (arrows). (B) Gliding tachyzoites were processed as for A. After fixation they were permeabilized and stained for either their micronemes, using the mouse monoclonal antibody anti-MIC1, or for their dense granules, using the mouse monoclonal antibody anti-GRA3. In both cases, the secondary anti-mouse antibody was conjugated to Alexa-488, and the staining is shown in green. After removal of the unbound conjugated antibody, the cells were stained for Toxofilin and for DNA as described above.