Figure 5.
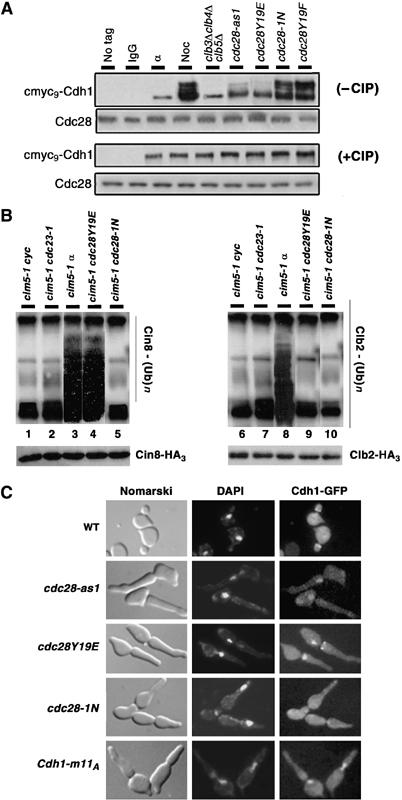
Phosphorylation status of Cdh1 determines its subcellular localization and Cin8 ubiquitylation. (A) Extracts were prepared from cmyc9-Cdh1 carrying WT cells grown in α factor (α) and nocodazole (Noc), and from clb3Δ clb4Δ clb5Δ GAL-CLB5, cdc28-as1, cdc28Y19E, cdc28-1N and cdc28Y19F arrested at their terminal points. Cdh1 was immunoprecipitated using anti-myc agarose beads, treated with and without alkaline phosphatase (CIP) and separated on a 10% SDS gel. Extracts from untagged WT strains (WT) as well as from nocodazole-arrested WT strain carrying cmyc9-Cdh1 immunoprecipitated with IgG beads (IgG) were used as negative controls. (B) cim5-1 cdc23-1 (lanes 2 and 7), cim5-1 cdc28Y19E (lanes 4 and 9), cim5-1 cdc28-1N (lanes 5 and 10) cells were synchronized in G1 with α factor and then released into fresh YPD medium at 37°C to reach their respective terminal phenotype. Since cim5-1 cdc28Y19E and cim5-1 cdc28-1N are synthetic lethal, they were kept alive by GAL-CDC28. Cycling (lanes 1 and 6) or α factor arrested (lanes 3 and 8) cim5-1 cells were used as controls. All strain carried endogenously tagged Cin8-HA3 or Clb2-HA3. Extracts were prepared, Clb2 and Cin8 were immunoprecipitated with anti-HA agarose beads, and ubiquitin conjagates were detected with anti-ubiquitin antibodies. Total amounts of Clb2-HA3 or Cin8-HA3 were detected using anti-HA antibodies. (C) WT, cdc28-as1, cdc28Y19E, cdc28-1N and WT overexpressing Cdh1-m11A (under the weak GALL promoter), all carrying Cdh1-GFP, were released from G1 arrest at their respective nonpermissive growth condition.
