Figure 1.
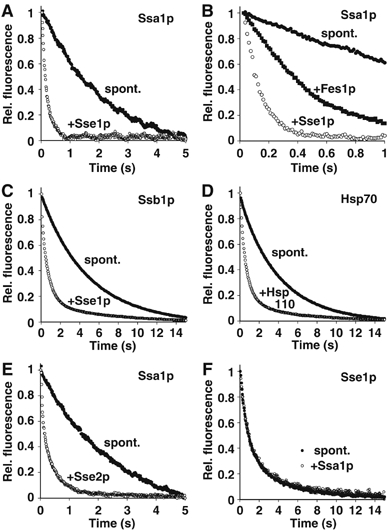
Hsp110 accelerates nucleotide exchange on Hsp70. (A) Accelerated dissociation of MABA-ADP from Ssa1p as monitored by stopped-flow fluorescence spectroscopy. Equimolar amounts of MABA-ADP and the indicated Hsp70 homologue were preincubated to form a complex, which was then mixed with an excess of ADP either in presence or absence of Sse1p. (B) Comparison of the efficiencies of nucleotide exchange in the presence of Sse1p and the canonical NEF, Fes1p. When applied at the same concentration (0.5 μM), Sse1p accelerates nucleotide release more efficiently than Fes1p. (C) Accelerated dissociation of MABA-ADP from Ssb1p. (D) The effect of human Hsp110 on the displacement of MABA-ADP from human Hsp70. (E) Release of MABA-ADP from Ssa1p by Sse2p. (F) Ssa1p does not trigger displacement of MABA-ADP from Sse1p. In all panels, effector-triggered and spontaneous dissociation is indicated by open and closed circles, respectively. Fes1p-triggered dissociation is indicated by closed squares. The molar ratio of effector to Hsp70 was 1:2 in all experiments.
