Abstract
Activated in immune responses, T lymphocytes differentiate into effector cells with potent immune function. CD28 is the most prominent costimulatory receptor for T-cell activation. However, absence of CD28 costimulation did not completely impair effector function of CD4 or CD8 T cells. Moreover, increasing number of costimulatory molecules are recently found on antigen-presenting cells to regulate T-cell activation. To understand the molecular mechanisms that determine T-cell function or tolerance, we have collectively examined the roles of positive and negative costimulatory molecules. Antigen-specific naïve CD4 and CD8 T cells, only when activated in the absence of both CD28 and ICOS pathways, were completely impaired in effector function. These tolerant T cells not only were anergic with profound defects in TcR signal transduction but also completely lacked expression of effector-specific transcription factors. T-cell tolerance induction in this system requires the action by negative costimulatory molecules; T-cell proliferation and function was partially restored by inhibiting PD-1, B7-H3 or B7S1. This work demonstrates that T-cell function or tolerance is controlled by costimulatory signals.
Keywords: costimulation, differentiation, IL-2, T cells, tolerance
Introduction
During infection, naïve pathogen-specific CD4 and CD8 T cells activated by antigen-presenting cells (APC) produce IL-2, undergo clonal expansion and subsequently differentiate into effector cells. T-cell differentiation is established and maintained via the induction and action of various transcriptional regulators. Th1 and CD8 effector generation requires T-bet and Eomes (Szabo et al, 2000; Pearce et al, 2003; Sullivan et al, 2003), whereas GATA-3 is the master regulator for Th2 differentiation (Zheng and Flavell, 1997).
In contrast to the robust responses to pathogen-associated antigens, presentation of self-antigens to T cells can result in T-cell tolerance, such as clonal deletion or anergy (Heath and Carbone, 2001; Steinman et al, 2003). Anergy was first discovered in Th1 clones when the TcR/CD3 complex was engaged in the absence of costimulation, which resulted in hyporesponsiveness to secondary TcR stimulation (Mueller et al, 1989; Schwartz, 2003). Based on this phenomenon, a two-signal model for T-cell activation was proposed, in which, in addition to the MHC–peptide complexes, a costimulatory ‘second' signal is required for T-cell activation (Mueller et al, 1989; Schwartz, 2003).
CD28 receptor on naïve T cells has long been regarded to deliver such signal (Janeway and Medzhitov, 2002). Naïve T cells costimulated with anti-CD28 exhibited greatly enhanced proliferation and IL-2 production. Consistently, mice deficient in CD28 or both of its ligands B7.1 and B7.2 (hereafter as B7-deficient mice) were severely impaired in CD4 T-cell proliferation (Shahinian et al, 1993; Borriello et al, 1997). In these mice, however, reduced levels of effector cytokines were still produced (Schweitzer and Sharpe, 1998; Tada et al, 1999; Girvin et al, 2000). Moreover, CD8 T-cell activation and function are less dependent on CD28 costimulation (Suresh et al, 2001). Thus, effector differentiation program of T cells was not completely impaired when naïve primary T cells were activated in the absence of CD28 pathway.
ICOS is the third member of the CD28 family expressed on activated T cells (Hutloff et al, 1999; Yoshinaga et al, 1999). Analysis of mice deficient in ICOS or its ligand, B7h, revealed that this pathway, although not globally required for CD4 T-cell activation and effector differentiation, regulates their selective effector function (Dong et al, 2001; Dong and Nurieva, 2003; Nurieva et al, 2003a, 2003b). Blockade of ICOS pathway did not result in impaired CD8 function in vivo (Bertram et al, 2002).
In addition to positive costimulation by CD28 and ICOS, other costimulators exist to inhibit T-cell activation. A second receptor for B7— CTLA4— is induced on activated T cells and serves as a negative regulator of T-cell activation and proliferation (Chambers and Allison, 1999). Mice deficient in CTLA4 died at neonatal stage owing to massive T-cell activation and infiltration into tissues. Although CD28 and CTLA4 are both recognized by the B7 molecules, the correlation of immune defects in B7 and CD28 knockout mice indicates that CD28 is the predominant B7 receptor during naïve T-cell activation. PD-1 is an inhibitory receptor expressed on activated T cells, which binds to PD-L1 (B7-H1) and PD-L2 (B7-DC) (Sharpe and Freeman, 2002). The spontaneous autoimmunity observed in PD-1-deficient mice indicates its critical function in immune tolerance (Sharpe and Freeman, 2002). B7-H3 is expressed in both lymphoid and nonlymphoid tissues (Chapoval et al, 2001; Sun et al, 2002). Mouse B7-H3 is constitutively expressed by professional APC and its expression on dendritic cells was further upregulated following LPS treatment (Prasad et al, 2004). Human and mouse B7-H3 bind to an unidentified receptor expressed on activated but not naïve T cells (Chapoval et al, 2001; Sun et al, 2002). Recent studies indicated that mouse B7-H3 is a negative regulator in T-cell activation; B7-H3 gene deletion or blockade with an antibody enhanced autoimmune responses in vivo (Suh et al, 2003; Prasad et al, 2004). B7S1/B7x/B7-H4 is the most recent addition in the B7 superfamily and is also widely expressed in lymphoid and nonlymphoid tissues (Prasad et al, 2003; Sica et al, 2003; Zang et al, 2003). B7S1 serves as a negative regulator of T cells by inhibiting their proliferation and IL-2 production (Prasad et al, 2003; Sica et al, 2003; Zang et al, 2003). Treatment of mice with an antagonistic antibody to B7S1 greatly enhanced experimental autoimmune encephalomyelitis (EAE) disease. Despite rich literature on the role of CTLA4 in T-cell tolerance, the contribution of new costimulatory pathways PD-1, B7-H3 and B7S1 to T-cell tolerance has not been well understood.
In this study, we assess the collective role of positive and negative costimulation in T-cell activation and functional differentiation. We first found that naïve CD4 and CD8 T cells activated in vitro in the absence of CD28 and ICOS engagement entered a state of tolerance that is characterized by anergy and complete absence of effector program. In addition, we found that generation of these tolerant T cells required negative costimulatory molecules PD-1, B7-H3 and B7S1. This work indicates that the fate of T cells is regulated by a combinatorial costimulatory signal.
Results
CD28 and ICOS compound deficiency completely impairs effector CD4 T-cell function
Reductionism approaches on T-cell costimulation have not fully elucidated the molecular signals that regulate T-cell immunity or tolerance. We thus attempted to examine the effects of combined mutations/blockade of costimulatory pathways in T-cell activation and function. CD28 and ICOS doubly deficient animals were recently reported to exhibit greater humoral and cytokine defects than single knockout mice (Suh et al, 2004; Park et al, 2005; Vidric et al, 2005); whether T cells were permanently toleralized in these systems was however not characterized. We first bred ICOS-deficient mice with OT-II TcR transgenic mice. CD4 T cells from these mice or their wild-type OT-II controls, all with similar CD62LhiCD44-CD25- naïve phenotypes (data not shown), were activated with Ova peptide presented by either wild-type or B7-deficient splenic APC. After 48 h of activation, ICOS+/+ and ICOS−/− Vα2+ transgenic T cells activated in the presence and absence of B7 all upregulated the activation markers CD69 and CD25, indicating their similar recognition of the antigen (Figure 1A). On the other hand, antigen-driven proliferation measured by CFSE division showed that T cells activated in the absence of single or double costimulatory pathways exhibited different degrees of reduction in proliferation (Figure 1B). Cells activated without B7 and ICOS costimulation were most impaired in proliferation and only underwent approximately 2–3 divisions.
Figure 1.
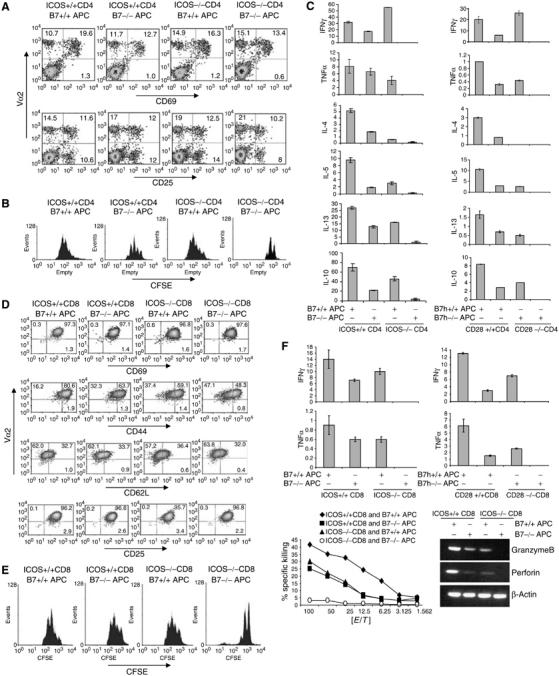
Defective CD4 and CD8 T-cell activation in vitro in the absence of ICOS and B7 costimualation. (A) CD4+ T cells purified from ICOS +/+ or ICOS−/− OT-II mice were treated with Ova peptide in the presence of irradiated B7+/+ or B7−/− APC for 2 days, and stained with antibodies to Vα2 (PE) and CD69 or CD25 (FITC). (B) CFSE-labeled ICOS +/+ or ICOS−/− OT-II cells were activated as in (A) for 3 days. Cells were stained with Vα2-PE antibody and analyzed by FACS. Histograms represent CFSE intensity of Vα2+ populations. (C) ICOS +/+ or ICOS−/− OT-II cells were activated with Ova peptide and irradiated B7+/+ or B7−/− APC for 4 days. CD28 +/+ or CD28−/− CD4+ cells from TEa mice were treated with Ea peptide in the presence of B7h+/+ or B7h−/− APC. After 4 days, differentiated T cells were restimulated with anti-CD3 for 24 h, and effector cytokine production was measured by ELISA. All cytokines are at ng/ml. (D) CD8+ T cells from ICOS+/+ or ICOS−/− OT-I mice were stimulated with SIINFEKL peptide and irradiated B7+/+ or B7−/− APC for 2 days. Cells were stained with antibodies to Vα2 (PE) and the activation markers CD69, CD25, CD62L or CD44 (FITC). (E) CFSE-labeled ICOS+/+ and ICOS−/− OT-I cells were stimulated as in (D). CFSE division was analyzed by FACS on day 3 after T-cell activation. Cells were stained with anti-Vα2 antibodies, and analyzed by FACS. Histograms represent CFSE intensity of Vα2+ populations. (F) ICOS+/+ or ICOS −/− CD8 OT-I cells were treated with SIINFEKL peptide, and CD28+/+ or CD28 −/− CD8 2C cells with SIY peptide for 7 days in the presence of irradiated B7+/+ or B7−/− APC. Differentiated T cells were then restimulated with plate-bound anti-CD3 for 24 h and effector cytokine production was measured by ELISA. All parameters in Y-axis are ng/ml. For the CTL assay, OT-I T cells differentiated as above were incubated for 6 h with SIINFEKL peptide-pulsed EL-4 syngeneic targets. Specific lysis was calculated and shown. Differentiated OT-I T cells were analyzed for granzime B, perforin and β-actin mRNA expression by RT–PCR.
At 4–7 days after activation, only Vα2+ T cells remained in all culture (data not shown), suggesting that antigen-specific T cells activated in the absence of CD28 and ICOS costimulation could survive over time. In fact, we did not observe any major difference in cell death among different culture conditions (Supplementary Figure 1A). To analyze the effector T-cell function that resulted from different stimuli, we recovered T cells 4 days after activation and restimulated them with plate-bound anti-CD3 to examine their cytokine expression. We found that B7 or ICOS deficiency alone resulted in defects in effector cytokine expression (Figure 1C), consistent with the literature. Without B7, all cytokines were reduced but still well above the detectable levels. ICOS deficiency, as we previously reported, had most significant impact on IL-4 expression (Dong et al, 2001; Nurieva et al, 2003a) (Figure 1C). Most strikingly, OT-II cells activated in the absence of both B7 and ICOS produced hardly any detectable effector cytokine (Figure 1C). Restimulation with anti-CD3 and anti-CD28 did not restore cytokine production in these T cells (Supplementary Figure 2A). Therefore, CD4 T cells activated only in the absence of both costimulations were globally impaired in their effector function.
We also stimulated OT-II cells with wild-type APC or those isolated from mice deficient in B7.1, B7.2 and B7h. We previously showed that B7h and ICOS are the only ligand and receptor for each other and mice deficient in either gene exhibited identical phenotypes (Nurieva et al, 2003b). OT-II cells activated by B7−/−B7h−/− APC were also found completely deficient in effector function (data not shown), similar to ICOS−/−OT-II cells activated in the presence of B7−/− APC (Figure 1C). As B7.1 and B7.2 can bind to CD28 as well as CTLA4, we also analyzed wild-type or CD28-deficient TEa TcR transgenic T cells activated in the presence of wild-type or B7h−/− APC (Figure 1C). CD28−/− TEa cells activated by B7h−/− APC, similar to ICOS−/−OT-II cells activated with B7−/− APC and OT-II cells activated with B7−/−B7h−/− APC, exhibited complete absence of effector function (Figure 1C). Therefore, the phenotypes we observed above with B7−/− APC were consistent with defective CD28 signaling. By use of these three in vitro systems, we conclude that naïve T-cell activation in the absence of CD28 and ICOS engagement resulted in complete impairments in effector CD4 T-cell function.
Generation of CD8 T-cell effector function requires CD28 and ICOS
Deficiency in CD28 or ICOS did not result in significant defects in CD8 T-cell activation and function. To determine the redundancy of CD28 and ICOS pathways in CD8 cell activation and function, we crossed ICOS-deficient mice with OT-I TcR transgenic mice. ICOS+/+ and ICOS−/− OT-I cells, all with naïve CD62LhiCD44-CD25- phenotype (data not shown), were activated with wild-type or B7-deficient APC in the presence of SIINFEKL peptide. Similar to the above observation on OT-II cells, OT-I cells activated under all conditions for 48 h exhibited similar upregulation of activation markers CD69 and CD25, and downregulation of CD62L (Figure 1D). CD44 upregulation was moderately reduced in OT-I cells activated in the absence of B7 and/or ICOS (Figure 1D). Activation in the absence of B7 and/or ICOS resulted in modest reduction of proliferation as measured by CFSE division (Figure 1E). Naïve OT-I cells activated in the absence of both CD28 and ICOS costimulation were most defective in proliferation and only underwent 2–3 divisions (Figure 1E).
Similar to OT-II cells, OT-I cells activated in the absence of both B7 and ICOS pathways survived 1 week after activation with no increased apoptosis (Supplementary Figure 1A). We examined their effector function after restimulation with anti-CD3. Absence of one pathway during priming resulted in only moderately reduced expression of cytokines IFNγ and TNFα (Figure 1F) and cytolytic enzymes Granzyme B and perforin (Figure 1F) in response to anti-CD3. Most significantly, deficiencies in both costimulation led to global impairment in cytokine and cytolytic enzyme expression, and these T cells could not kill peptide-pulsed EL4 cells (Figure 1F). Similarly, we also found CD28−/− 2C CD8 T cells, after activation with B7h−/− APC, exhibited complete effector cytokine deficiency (Figure 1F) in response to TcR stimulation. Moreover, CD28 costimulation did not restore proliferation and cytokine production of CD8 T cells that had been initially stimulated in the absent of CD28 and ICOS signals (Supplementary Figure 2B). Therefore, CD8 cell activation in the absence of both CD28 and ICOS also were completely deficient in effector function.
Signaling and transcriptional defects in tolerant T cells
To further characterize the defects in tolerant T cells that were activated in the absence of CD28 and ICOS engagement, we first activated OT-II cells in the presence of wild-type or B7−/−B7h−/− APC and then restimulated them with different doses of anti-CD3. T cells activated without CD28 and ICOS engagement exhibited greatly reduced proliferation (Figure 2A) without increased cell death (Supplementary Figure 1B). Similarly, CD28−/− TEa cells activated by B7h−/− APC exhibited hypoproliferative response to TcR restimulation (Figure 2A). They therefore were anergic to TcR/CD3 restimulation.
Figure 2.
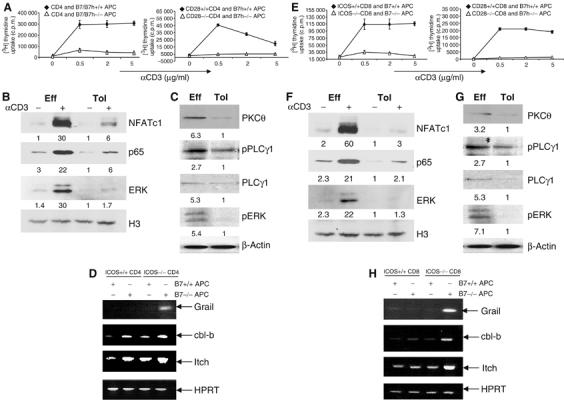
Signaling defects in T cells activated in the absence of both CD28 and ICOS costimulation. (A) CD4 OT-II cells were treated with Ova peptide in the presence of irradiated wild-type or B7−/−B7h−/− APC. CD28+/+ or CD28−/− TEa cells were treated with Ea peptide in the presence of irradiated B7h+/+ or B7h −/− APC. After 4 days, T cells were restimulated with different concentrations of anti-CD3. Proliferation was assayed 24 h after treatment by adding [3H]thymidine to the culture for the last 8 h. (B) Effector (Eff) and tolerant (Tol) OT-II cells were generated as described in (A). These cells were restimulated with plate-bound anti-CD3 for 4 h. Nuclear extracts prepared from unrestimulated and restimulated with anti-CD3 cells were probed with ERK, NFATc1 and NFκB p65 antibodies. Histon 3 (H3) was used as loading control. Relative Western blotting signals were inducted. (C) Eff and Tol CD4 cells were restimulated were restimulated with anti-CD3 for 30 min and cytoplasmic fractions were probed with PKCθ and PLCγ1 antibodies and phosphospecific antibodies against PLCγ1 and ERK. β-Actin was used as loading control. Relative Western blotting signals were inducted. (D) Grail, cbl-b, Itch and HPRT mRNA expression in restimulated effector and tolerant CD4 T cells (as in B) was analyzed by RT–PCR. (E) ICOS+/+ or ICOS −/− OT-I cells were activated with SIINFEKL peptide plus irradiated B7+/+ or B7−/− APC. CD8 cells from CD28+/+ or CD28 −/− 2C mice were treated with SIY peptide in the presence of irradiated B7h+/+ or B7h−/− APC. After 4 days, T cells were restimulated with different concentrations of plate-bound anti-CD3. Proliferation was assayed 24 h after restimulation by adding [3H]thymidine to the culture for the last 8 h. (F) Eff and Tol CD8 cells were generated as described in Figure 1F. These cells were restimulated with plate-bound anti-CD3 for 4 h. Nuclear extracts prepared from nonrestimulated and restimulated cells were probed with ERK, NFATc1 and NFκB p65 antibodies. H3 was used as loading control. Relative Western blotting signals were inducted. (G) Eff and Tol CD8 cells were restimulated with anti-CD3 for 30 min and cytoplasmic fractions were probed with PKCθ and PLCγ1 antibodies and phosphospecific antibodies against PLCγ1 and ERK. β-Actin was used as loading control. Relative Western blotting signals were inducted. (H) Grail, cbl-b, Itch and HPRT mRNA expression in restimulated effector and tolerant CD8 T cells (as in F) was analyzed by RT–PCR.
We next examined TcR signal transduction in CD4 T cells preactivated in the absence of CD28 and ICOS costimulation. AP1, NF-κB and NFAT are three major signaling pathways through which TcR regulates gene transcription. We thus analyzed activation MAP kinase, NF-κB and NFAT pathways after 4-h anti-CD3 treatment of activated T cells. Our results indicated that in tolerant CD4 T cells primed in the absence of both CD28 and ICOS pathways, nuclear accumulation of NFATc1, p65 and ERK was greatly impaired (Figure 2B and C). Degradation of IκBα was significantly reduced in tolerant T cells compared to the effector T cells, further supporting an NF-κB defect in these cells (Supplementary Figure 3A). The expression of ERK and p65 was not significantly altered in effector and tolerant T cells before or after restimulation (Supplementary Figure 3A). NFATc1 expression was enhanced in effector T cells upon restimulation and tolerant T cells expressed less NFATc1 protein compared to effector cells (Supplementary Figure 3A). However, this difference was not so dramatic as the reduction of nuclear NFATc1 in tolerant T cells. Therefore, these cells had a global defect in TcR-initiated signaling and gene regulation program.
PLCγ1 and PKCθ were recently found as targets of ubiquitination-dependent protein degradation in ionomycin-induced anergic T cells (Heissmeyer and Rao, 2004; Mueller, 2004). Consistent with this report (Heissmeyer et al, 2004), we found that expression of these two crucial components in TcR signaling as well as the amount of phosphorylated PLCγ1 was greatly reduced in CD4 T cells activated in the absence of CD28 and ICOS costimulation (Figure 2C). Expression of two other E3 ubiquitin ligases cbl-b and Itch was also enhanced in T cells primed without CD28/ICOS costimulations (Figure 2D). Most strikingly, CD4 T cells, only after activation in the absence of both CD28 and ICOS, expressed Grail (Figure 2D), an E3 ubiquitin ligase previously reported to be abundantly expressed in anergic Th1 cells (Anandasabapathy et al, 2003).
Similar to OT-II cells, OT-I cells activated in the absence of both B7 and ICOS pathways exhibited normal cell death (Supplementary Figure 1B) but greatly reduced proliferation (Figure 2E) when restimulated with anti-CD3. CD28−/− 2C CD8 T cells, after activation with B7h−/− APC, also proliferated poorly (Figure 2E) in response to anti-CD3 restimulation. Furthermore, activation of ERK, NFAT and NF-κB pathways were greatly impaired in OT-I cells activated in the absence of B7/ICOS (Figure 2F, Supplementary Figure 3B). These cells, similar to their OT-II counterparts (Figure 2C), also exhibited deficiencies in PKCθ and PLCγ1 expression (Figure 2G). The expression of Grail, cbl-b and Itch were highly upregulated only in OT-I cells activated in the absence of both B7 and ICOS costimulation (Figure 2H).
Thus, T cells activated without CD28 and ICOS restimulation exhibited profound TcR signaling defects. To test whether the complete absence of effector function could result from these defects, we restimulated OT-II and OT-I T cells activated with wild-type or B7−/−B7h−/− APC with PMA/ionomycin and measured their proliferation and cytokine expression. PMA/ionomycin stimulation significantly increased the proliferation of anergic T cells to levels comparable to effector T cells (Figure 3A). However, these cells still exhibited an IFNγ deficiency by intracellular cytokine expression after PMA/ionomycin stimulation (Figure 3A). This suggests that T cells activated in the absence of CD28 and ICOS engagements may have some fundamental developmental defect. We thus performed sensitive RT–PCR analysis to examine the expression of master transcription regulators for CD4 and CD8 effector differentiation. We found that OT-II T cells, only when activated in the absence of both CD28 and ICOS, did not express T-bet (Szabo et al, 2000) or GATA-3 (Zheng and Flavell, 1997), master regulators for Th1 or Th2 lineage commitment (Figure 3B, Supplementary Figure 4A), respectively. On the other hand, OT-I T cells, only when activated in the absence of both CD28 and ICOS costimulation, also completely lacked expression of T-bet and Eomes (Figure 3B, Supplementary Figure 4B), transcription factors required for CD8 effector function (Pearce et al, 2003; Sullivan et al, 2003). As these factors are required to establish the chromatin configuration for effector gene expression and effector function, we believe that effector transcription programs failed to be established or maintained in tolerant T cells activated in the absence of CD28 and ICOS.
Figure 3.
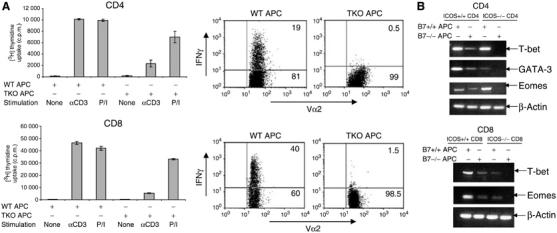
Effector defects in T cells activated in the absence of both CD28 and ICOS costimulation. (A) CD4 OT-II cells or CD8 OT-I cells were treated with Ova peptide or SIINFEKL peptide in the presence of irradiated wild-type or B7−/−B7h−/− APC. After 4 days, T cells were restimulated with anti-CD3 (5 μg/ml) or PMA/Ionomycin. Proliferation was assayed 24 h after restimulation by adding [3H]thymidine to the culture for the last 8 h. For cytokine measurement, CD4 OT-II cells or CD8 OT-I cells were treated as described above. After 5 days, T cells were restimulated with PMA/Ionomycin for 5 h. Intracellular staining of IFNγ was performed on gated Vα2+ T cells, and then analyzed by FACS. (B). T-bet, GATA3, Eomes and β-actin mRNA expression in effector and tolerant CD4 T cells (as in Figure 1C) was analyzed by RT–PCR. Effector and tolerant CD8+ T cells (from 1F) were analyzed for T-bet, Eomes and β-actin mRNA expression by RT–PCR.
Regulation of T-cell differentiation by negative costimulatory molecules
Activation of naïve T cell in the absence of CD28 and ICOS resulted in their anergy and effector deficiencies, suggesting that the ‘second' signal can be delivered by CD28 or ICOS. However, previous work indicated that naïve T cells receiving only TcR stimulation were not anergized (Ragazzo et al, 2001; Andris et al, 2004), suggesting that additional signal on APC may be required for T-cell tolerization. Negative costimulatory molecules PD-1, B7-H3 and B7S1 have been shown to regulate T-cell activation thresholds, and loss of their action often resulted in greater susceptibility to autoimmune diseases (Sharpe and Freeman, 2002; Prasad et al, 2003, 2004; Suh et al, 2003). Recently, CD8+ T cells specific for a chronic viral antigen were found to be ‘exhausted' and expressed higher levels of PD-1 (Barber et al, 2006). Similarly, we found that tolerant T cells, especially CD4+, appeared to express increased levels of PD-1, whereas OX40 expression was not significantly altered (Supplementary Figure 5). We thus examined whether the inhibitory molecules play any role in the induction of T-cell tolerance. OT-II cells were first activated in the presence of wild-type or B7−/−B7h−/− APC with or without antagonistic antibodies to PD-1 (clone J43, eBioscience) (Agata et al, 1996), B7-H3 (clone 110) (Prasad et al, 2004) or B7S1 (clone 54) (Prasad et al, 2003). All these antibodies enhanced proliferation and IL-2 production by OT-II cells activated with wild-type APC (Supplementary Figure 6A). They also restored the proliferation of OT-II cells activated with B7−/−B7h−/− APC to similar levels as OT-II cells activated with wild-type APC (Supplementary Figure 6A). As a result of blocking PD-1, B7-H3 or B7S1, OT-II cells activated in the absence of B7 and B7h costimulation for 4 days exhibited increased proliferative responses to anti-CD3 restimulation (Figure 4A), and more significantly, these cells produced effector cytokines (Figure 4B). Interestingly, B7S1 blocking was more potent in restoring Th2 cytokines IL-4, -5, and -13, compared with the other two antibodies (Figure 4B). By examining factors upregulated or downregulated in tolerant T cells, we found that blocking B7S1, B7-H3 or PD-1 restored expression of GATA-3, T-bet and Eomes and downregulated Grail, Itch and Cbl-b expression (Figure 4C and D, Supplementary Figure 4A).
Figure 4.
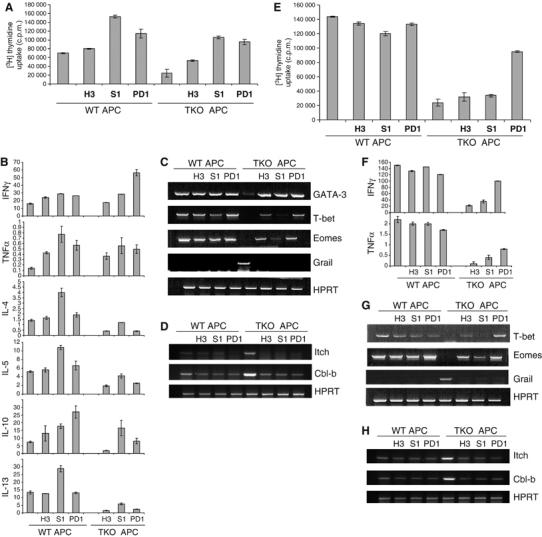
Negative costimulatory molecules regulate tolerance induction of CD4 and CD8 T cells. (A) OT-II cells were treated with Ova peptide in the presence of irradiated wild-type (WT) or B7−/−B7h−/− (TKO) APC with a control rat IgG or a blocking antibody to B7S1 (S1) or B7H3 (H3) or PD1. At 4 days after activation, T cells were restimulated with plate-bound anti-CD3. Proliferation was assayed 24 h after treatment by adding [3H]thymidine to the culture for the last 8 h. (B) CD4 cells activated as in (A) were restimulated with plate-bound anti-CD3 for 24 h and cytokine production was measured by ELISA. All parameters indicated in Y-axis are at ng/ml. (C) Differentiated OT-II T cells were analyzed for GATA-3, T-bet, Eomes, Grail and HPRT mRNA expression by RT–PCR. (D) Differentiated OT-II T cells were analyzed for Itch, Cbl-b and HPRT mRNA expression by RT–PCR. (E) OT-I cells were treated with SIINFEKL peptide in the presence of irradiated WT or B7−/−B7h−/− (TKO) APC with a control rat IgG or a blocking antibody to B7S1 (S1) or B7H3 (H3) or PD1. At 6–7 days after activation, T cells were restimulated with plate-bound anti-CD3. Proliferation was assayed 24 h after treatment by adding [3H]thymidine to the culture for the last 8 h. (F) CD8 cells activated as in (E) were restimulated with plate-bound anti-CD3 for 24 h and cytokine production was measured by ELISA. All parameters indicated in Y-axis are at ng/ml. (G) Differentiated OT-I T cells were analyzed for T-bet, Eomes, Grail and HPRT mRNA expression by RT–PCR. (H) Differentiated OT-I T cells were analyzed for Itch, Cbl-b and HPRT mRNA expression by RT–PCR.
We then examined the effect of PD-1, B7-H3 and B7S1 blockade when OT-I cells were activated with B7−/−B7h−/− APC. They did not alter significantly the proliferation and IL-2 production by CD8 cells activated with wild-type APC or B7−/−B7h−/− APC (Supplementary Figure 6B). Only anti-PD-1 strongly restored proliferative responses upon restimulation and was most potent in induction of effector cytokine production by restimulated CD8 T cells (Figure 4E and F). On the other hand, all antibodies upregulated Eomes; anti-B7S1 and anti-PD-1 restored T-bet expression as well. Grail, Itch and Cbl-b expression was downregulated by all blocking antibodies (Figure 4G and H, Supplementary Figure 4B). Therefore, our data indicate that T-cell tolerance resulting from B7 and ICOS blockade required the function of PD-1, B7-H3 and B7S1.
IL-2 inhibits induction of T-cell tolerance
The above data demonstrated that physiological APC present antigen in the context of both positive and negative costimulatory molecules, which determine the fate of antigen-specific T cells. Considering that CD28 and ICOS enhance, while B7-H3, B7S1 and PD-1 inhibit IL-2 production (Carter et al, 2002; Prasad et al, 2003, 2004), we tested whether T-cell tolerance and immunity is regulated by IL-2. We found that OT-II cells activated by B7−/−B7h−/− APC in the presence anti-B7-H3, -B7S1, or –PD-1 and OT-I cells activated by the same APC in the presence of PD-1 exhibited increased levels of IL-2 compared to cells treated with a control antibody (Supplementary Figure 6A and B). In addition, OT-II and OT-I cells were activated with wild-type and B7−/−B7h−/− APC in the absence or presence of exogenous IL-2, and the resulting T cells were restimulated before analysis of their phenotypes. T cells activated with B7−/−B7h−/− APC exhibited hypoproliferation and effector defects (Figure 5A and C). Addition of exogenous IL-2 enhanced proliferative responses and restored cytokine expression in CD4 and CD8 cells (Figure 5A and C). Intracellular cytokine staining revealed that OT-II or OT-I cells treated with B7−/−B7h−/− APC in the presence of IL-2 were able to express IFNγ (Supplementary Figure 7A and B). Consistently, when activated with B7−/−B7h−/− APC and exogenous IL-2, OT-II T cells upregulated T-bet, Eomes and GATA-3 and downregulated Grail expression (Figure 5B), and OT-I cells treated in the same way upregulated T-bet and Eomes and downregulated Grail (Figure 5D). Thus, IL-2 inhibits T-cell tolerance induction. Positive and negative costimulatory molecules may function, in part, by regulating the levels of IL-2 production.
Figure 5.
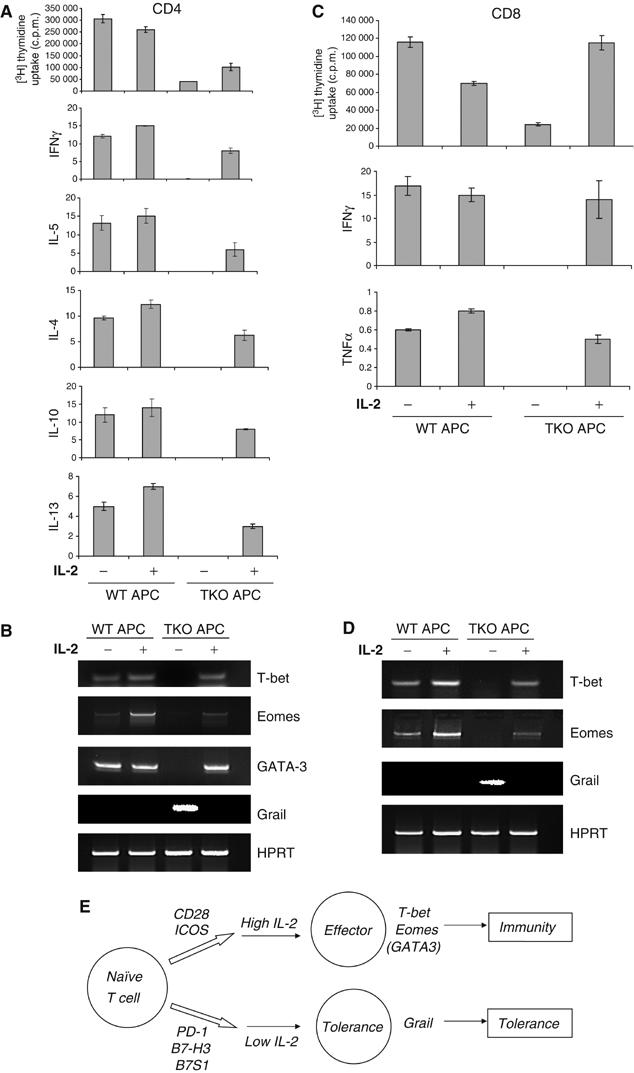
IL-2 regulates T-cell tolerance and immunity. (A–D) CD4 OT-II cells (A, B) or CD8 OT-I cells (C, D) were treated with Ova peptide or SIINFEKL peptide, respectively, in the presence of irradiated wild-type (WT) or B7−/−B7h−/− (TKO) APC with or without IL-2. After 4 days for CD4 (A) and 6–7 days for CD8 cells (C), T cells were restimulated with plate-bound α-CD3 to measure T-cell proliferation and effector cytokine (IFN-γ, IL-4, -5, -10, -13) production. Activated OT-II (B) or OT-I (D) cells were analyzed for T-bet, Eomes, Grail and HPRT mRNA expression by RT–PCR. (E) T-cell immunity or tolerance is regulated by a combinatorial costimulation signal. At resting states, when T cells encounter APC, negative costimulatory molecules PD-1, B7-H3 and B7S1 function to inactivate T cells. During infection, CD28 and ICOS ligands are upregulated on APC, which drives T cells to differentiate into effector T cells.
Discussion
In this study, we have collectively examined the positive and negative costimulatory molecules in CD4 and CD8 T-cell activation and effector function. We found that naïve T cells, after activation in the absence of CD28 and ICOS, were completely impaired in effector differentiation and that negative costimulatory molecules were required for tolerance induction.
Previous work has demonstrated that deficiency in either B7/CD28 or B7h/ICOS resulted in defective immune responses; however, absolute T-cell tolerance or anergy was not observed. In our current work, we first found that in the presence of specific peptides presented on physiological APC, antigen-specific T cells could respond by upregulating activation markers and undergo 2–3 cell divisions in the absence of CD28 and ICOS costimulation. These T cells activated in the absence of costimulation could persist in culture for 4–7 days at least, but had characteristics of anergic cells—hyporesponsiveness to TcR/CD3 restimulation. Biochemical characterization revealed that these cells were deficient in TcR signal transduction leading to gene transcription. TcR activation of MAP kinase, NFAT and NF-κB pathways was greatly inhibited in these cells (Figure 2B and F, Supplementary Figure 3A and B). As these anergic cells proliferated well to PMA/ionomycin stimulation, it is likely that they had deficiencies in early events of TcR signaling. In support of this idea, they expressed reduced levels of PKCθ and PLCγ1 (Figure 2C and G), recently identified targets of ubiquitin-regulated degradation machinery in Ca2+-induced anergic cells (Heissmeyer et al, 2004). On the other hand, expression of Grail, cbl-b and Itch ubiquitin ligases was significantly induced in these cells (Figure 2D and H). Therefore, these CD4 and CD8 cells shared some characteristics with anergic Th1 cells characterized previously by others (Schwartz, 2003; Mueller, 2004).
Additionally and most interestingly, the anergic cells generated by activating naïve antigen-specific T cells in the absence of costimulation did not exhibit any effector function. The CD4 anergic T cells did not produce any effector cytokine upon restimulation (Figure 1C, Supplementary Figure 2A), whereas CD4 cells activated in the absence of B7 or ICOS produced low but detectable level of effector cytokines. The anergic CD8 T cells did not express cytokines and cytolytic enzymes, and could not kill target cells (Figure 1F, Supplementary Figure 2B). We found that they only activated in the absence of both CD28/ICOS, T cells did not express transcription factors that regulate effector differentiation and cytokine expression (Figure 3B, Supplementary Figure 4A and B). PMA/ionomycin could restore proliferation but not cytokine expression in anergic T cells (Figure 3A). These results indicate that the global impairments in effector function in the tolerant cells was not due to simple TcR signaling defects but rather intrinsic absence of an effector gene expression program. Thus when antigen-specific naïve T cells were activated in the absence of CD28 and ICOS costimulation, instead of differentiating into effector cells, they likely developed into stably tolerized T cells with both TcR signaling and gene transcription defects. Absence of B7 or ICOS costimulation resulted only in defective immune functions, but absolute T-cell tolerance was not observed. T cells in these systems are thus fully inactivated ensured by signaling and transcriptional defects. Interestingly, T cells rendered anergic in vivo also exhibited effector deficiency that was not found in in-vitro-anergized T cells (Schwartz, 2003).
Our data indicate that the positive costimulation signal can be delivered by CD28 or ICOS. Recent expansion of the B7 family has revealed complexed negative regulation of T cells. Interestingly, tolerant T cells that were primed in the absence of CD28 and ICOS signaling exhibited increased expression of PD-1, but not OX40 (Supplementary Figure 5). In the current work, we have examined the requirements of PD-1, B7-H3 and B7S1 in the induction of T-cell tolerance caused by B7 and ICOS deficiencies. Blocking of any pathway led to partial breakdown of T-cell tolerance, as evidenced by increased proliferation and partially restored effector cytokine production. The involvement of negative costimulatory molecules in the induction of T-cell tolerance is consistent with previous reports that the TcR stimulation alone is not sufficient to cause anergy by naïve T cells (Ragazzo et al, 2001; Andris et al, 2004). How these molecules regulate the tolerance induction is not clear at this stage. Possibly, they could do so by regulating IL-2 production and cell cycle progression. In support of this idea, addition of exogenous IL-2 to T cells activated in the absence of CD28 and ICOS costimulations could reverse their anergy induction and generate effector function (Figure 5A and B). Notably, IL-2 did not restore TH cytokines to the same levels produced by effector T cells, suggesting IL-2-independent mechanisms. Thus the inhibitory molecules collectively deliver a negative signal to T cells, which results in T-cell dependency on CD28 or ICOS signaling for effector generation. It is also possible that these molecules individually have their functional specificity. For instance, blocking B7S1 could result in Th2 function, and anti-PD-1 was most potent in restoring CD8 cell function (Figure 4). Interestingly, combination of three blocking reagents resulted in reduced Th2 cytokines compared to cells treated only with anti-B7S1, but consistent with those treated with anti-PD-1 or anti-B7-H3 (Supplementary Figure 8). This may reflect the hierarchy of these costimulatory pathways; meanwhile, it is also possible that excessive amounts of IFNγ induced by anti-PD-1 and anti-B7-H3 inhibited Th2 differentiation.
In summary, our analysis indicates that the fate of naïve T cells, as they encounter an antigen, is determined by not a single ‘second' signal, but instead, rich combinatorial costimulation (Figure 5E). In the absence of positive costimulation mediated by CD28 and ICOS pathways, negative costimulatory molecules actively instruct T cells to develop into tolerant T cells characterized by inactivation of intrinsic signaling and transcriptional programs. Tolerant T cells expressed Grail but not transcription factors for effector T cells—T-bet and Eomes, as well as GATA-3 for CD4 T cells. On the other hand, during infection and inflammatory responses, B7 and B7h molecules are highly upregulated on APC, which overcomes the constitutive negative costimulation signals and results in a positive outcome of T-cell activation—extensive T-cell expansion and generation of effector function. In this case, T cells express transcription factors T-bet, Eomes and GATA-3 but not Grail, Itch or cbl-b.
Our work in demonstrating the costimulatory determination of the T-cell fate when they encounter an antigen has broad implication for immunotherapy. For instance, blockade of CD28 and ICOS pathways can be employed to generate antigen-specific immune tolerance. On the other hand, as lack of positive costimulation contributes to T-cell tolerance to self-antigens, inhibiting negative costimulation individually or collectively would benefit our battle against cancer.
Materials and methods
Mice
ICOS-deficient mice (Dong et al, 2001), backcrossed for six generations onto C57BL/6, were bred with OT-I or OT-II TCR transgenic mice. B7h knockout mice were previously analyzed on B6x129 F1 background (Nurieva et al, 2003b) and subsequently backcrossed for >6 generations onto C57BL/6. TEa TCR transgenic mice and CD28-deficient TEa mice were kindly provided by A Rudensky (University of Washington). CD80/CD86 doubly deficient mice on C57BL/6 background were purchased from Jackson Lab and bred with ICOS- and B7h-deficient animals.
T-cell stimulation
TcR transgenic CD4 or CD8 T cells from lymph nodes and spleens of 6- to 8-week-old mice were positively sorted using autoMACS (Miltenyi Biotec). Splenic APC from B6, B7−/−, B7h−/− and B7−/−B7h −/− mice were prepared by complement-mediated lysis of Thy1+ T cells. For analysis of T-cell activation, purified CD4+ or CD8+ T cells were stimulated in triplicates with different concentrations of peptide agonists in the presence of various APC. Proliferation was assayed on day 3 by adding [3H]thymidine to the culture for the last 8 h. CFSE (Molecular Probes, Eugene, OR) division was analyzed by FACS on day 3. To analyze effector function, OT-II or OT-I T cells (1 × 106 cells) were stimulated with Ova (5 μg/ml) or SIINFEKL peptide (0.01 μg/ml) in the presence of irradiated APC (1 × 106/ml). In some experiments, CD28 +/+ or CD28 −/− TEa or 2C T cells (1 × 106/ml) were stimulated with Ea peptide (1 μg/ml) or SIY peptide (1 μg/ml) in the presence of irradiated APC (1 × 106/ml). After 4 days for CD4 activation and 7 days for CD8, differentiated T cells were restimulated with plate-bound α-CD3 (5 μg/ml) for 24 h, and cytokine production was analyzed by ELISA (Pharmingen). Expression of transcription factors was analyzed by RT–PCR.
Cytotoxicity assay
ICOS+/+ or ICOS −/− CD8 OT-I cells were treated with SIINFEKL peptide in the presence of irradiated B7+/+ or B7−/− APC for 7 days. Differentiated CD8+ T cells were incubated for 6 h with 51Cr-labeled EL-4 syngeneic targets (3000 per reaction) pulsed with SIINFEKL peptide. Reactions were performed in triplicate at the indicated effector/target ratios. Nonspecific lysis was assessed without peptide loading; maximum lysis was measured by treating labeled targets with 10% SDS, and spontaneous lysis, in the absence of effectors. The percentage of specific lysis was calculated from the equation: ((experimental 51Cr release−nonspecific 51Cr release)/(maximum 51Cr release−nonspecific 51Cr release)) × 100%=percent-specific lysis.
Immunoblot analysis
Nuclear fraction of Th cells was prepared as described (Nurieva et al, 2003a). Whole Th cell lysates were prepared by lysing cells in triton lysis buffer. The amounts of protein were determined by Bio-Rad protein assay to ensure equal protein loading for Western blot analysis with antibodies to NFATc1, pERK, p65, PLCγ, pPLCγ, PKCθ and β-actin (Santa Cruz Biotechnology).
Supplementary Material
Supplementary Figure Legends
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Acknowledgments
We thank Dr Andrew Farr for his guidance in animal work, Dr Alexander Rudensky for TEa and CD28−/− TEa mice, Dr Mike Bevan for OT-I mice, Dr Steve Reiner for Eomes primer sequences, Dr Yong-Jun Liu and Caroline Bishop for careful reading of the manuscript and the entire Dong lab for their help and discussion. This work is supported in part by grants from the National Institute of Health (to CD, MK and XY). RN receives a postdoctoral fellowship from Arthritis Foundation and a Scientist Development Grant from American Heart Association, TN was supported by an NIH training grant and CD receives an Investigator award from the Cancer Research Institute and a Trust Fellowship from MD Anderson Cancer Center.
References
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T (1996) Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 8: 765–772 [DOI] [PubMed] [Google Scholar]
- Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L (2003) GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity 18: 535–547 [DOI] [PubMed] [Google Scholar]
- Andris F, Denanglaire S, de Mattia F, Urbain J, Leo O (2004) Naive T cells are resistant to anergy induction by anti-CD3 antibodies. J Immunol 173: 3201–3208 [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R (2006) Restoring function in exhausted CD8T cells during chronic viral infection. Nature 439: 682–687 [DOI] [PubMed] [Google Scholar]
- Bertram EM, Tafuri A, Shahinian A, Chan VS, Hunziker L, Recher M, Ohashi PS, Mak TW, Watts TH (2002) Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol 32: 3376–3385 [DOI] [PubMed] [Google Scholar]
- Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH (1997) B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity 6: 303–313 [DOI] [PubMed] [Google Scholar]
- Carter LL, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM (2002) PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol 32: 634–643 [DOI] [PubMed] [Google Scholar]
- Chambers CA, Allison JP (1999) Costimulatory regulation of T cell function. Curr Opin Cell Biol 11: 203–210 [DOI] [PubMed] [Google Scholar]
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L (2001) B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2: 269–274 [DOI] [PubMed] [Google Scholar]
- Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA (2001) ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 409: 97–101 [DOI] [PubMed] [Google Scholar]
- Dong C, Nurieva RI (2003) Regulation of immune and autoimmune responses by ICOS. J Autoimmun 21: 255–260 [DOI] [PubMed] [Google Scholar]
- Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD (2000) A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol 164: 136–143 [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR (2001) Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol 19: 47–64 [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A (2004) Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol 5: 255–265 [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Rao A (2004) E3 ligases in T cell anergy—turning immune responses into tolerance. Sci STKE 2004: pe29. [DOI] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Krocsek RA (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397: 263–266 [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216 [DOI] [PubMed] [Google Scholar]
- Mueller DL (2004) E3 ubiquitin ligases as T cell anergy factors. Nat Immunol 5: 883–890 [DOI] [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK, Schwartz RH (1989) Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol 7: 445–480 [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C (2003a) Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity 18: 801–811 [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Mai XM, Forbush K, Bevan MJ, Dong C (2003b) B7h is required for T cell activation, differentiation, and effector function. Proc Natl Acad Sci USA 100: 14163–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, Wang Y, Hood L, Zhu Z, Tian Q, Dong C (2005) A distinct lineage of CD4T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6: 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL (2003) Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302: 1041–1043 [DOI] [PubMed] [Google Scholar]
- Prasad DV, Richards S, Mai XM, Dong C (2003) B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity 18: 863–873 [DOI] [PubMed] [Google Scholar]
- Prasad DVR, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C (2004) Mouse B7-H3 is a negative regulator of T cells. J Immunol 173: 2500–2506 [DOI] [PubMed] [Google Scholar]
- Ragazzo JL, Ozaki ME, Karlsson L, Peterson PA, Webb SR (2001) Costimulation via lymphocyte function-associated antigen 1 in the absence of CD28 ligation promotes anergy of naive CD4+ T cells. Proc Natl Acad Sci USA 98: 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH (2003) T cell anergy. Annu Rev Immunol 21: 305–334 [DOI] [PubMed] [Google Scholar]
- Schweitzer AN, Sharpe AH (1998) Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol 161: 2762–2771 [PubMed] [Google Scholar]
- Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW (1993) Differential T cell costimulatory requirements in CD28-deficient mice. Science 261: 609–612 [DOI] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ (2002) The B7-CD28 superfamily. Nat Rev Immunol 2: 116–126 [DOI] [PubMed] [Google Scholar]
- Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L (2003) B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity 18: 849–861 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21: 685–711 [DOI] [PubMed] [Google Scholar]
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW (2003) The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 4: 899–906 [DOI] [PubMed] [Google Scholar]
- Suh W-K, Tafuri A, Berg-Brown NN, Shahinian A, Plyte S, Duncan GS, Okada H, Wakeham A, Odermatt B, Ohashi PS, Mak TW (2004) The inducible costimulator plays the major costimulatory role in humoral immune responses in the absence of CD28. J Immunol 172: 5917–5923 [DOI] [PubMed] [Google Scholar]
- Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH (2003) Antigen-driven effector CD8T cell function regulated by T-bet. Proc Natl Acad Sci USA 100: 15818–15823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C (2002) Characterization of mouse and human B7-H3 genes. J Immunol 168: 6294–6297 [DOI] [PubMed] [Google Scholar]
- Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R (2001) Role of CD28–B7 interactions in generation and maintenance of CD8T cell memory. J Immunol 167: 5565–5573 [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH (2000) A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669 [DOI] [PubMed] [Google Scholar]
- Tada Y, Nagasawa K, Ho A, Morito F, Ushiyama O, Suzuki N, Ohta H, Mak TW (1999) CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol 162: 203–208 [PubMed] [Google Scholar]
- Vidric M, Suh W-K, Dianzani U, Mak TW, Watts TH (2005) Cooperation between 4-1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J Immunol 175: 7288–7296 [DOI] [PubMed] [Google Scholar]
- Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G (1999) T-cell co-stimulation through B7RP-1 and ICOS. Nature 402: 827–832 [DOI] [PubMed] [Google Scholar]
- Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP (2003) B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA 100: 10388–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Flavell RA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4T cells. Cell 89: 587–596 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure Legends
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8


