Figure 2.
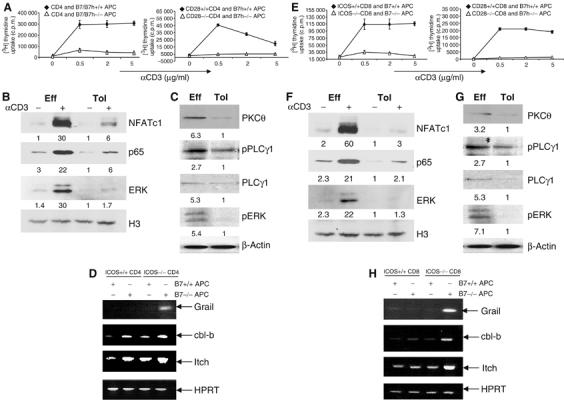
Signaling defects in T cells activated in the absence of both CD28 and ICOS costimulation. (A) CD4 OT-II cells were treated with Ova peptide in the presence of irradiated wild-type or B7−/−B7h−/− APC. CD28+/+ or CD28−/− TEa cells were treated with Ea peptide in the presence of irradiated B7h+/+ or B7h −/− APC. After 4 days, T cells were restimulated with different concentrations of anti-CD3. Proliferation was assayed 24 h after treatment by adding [3H]thymidine to the culture for the last 8 h. (B) Effector (Eff) and tolerant (Tol) OT-II cells were generated as described in (A). These cells were restimulated with plate-bound anti-CD3 for 4 h. Nuclear extracts prepared from unrestimulated and restimulated with anti-CD3 cells were probed with ERK, NFATc1 and NFκB p65 antibodies. Histon 3 (H3) was used as loading control. Relative Western blotting signals were inducted. (C) Eff and Tol CD4 cells were restimulated were restimulated with anti-CD3 for 30 min and cytoplasmic fractions were probed with PKCθ and PLCγ1 antibodies and phosphospecific antibodies against PLCγ1 and ERK. β-Actin was used as loading control. Relative Western blotting signals were inducted. (D) Grail, cbl-b, Itch and HPRT mRNA expression in restimulated effector and tolerant CD4 T cells (as in B) was analyzed by RT–PCR. (E) ICOS+/+ or ICOS −/− OT-I cells were activated with SIINFEKL peptide plus irradiated B7+/+ or B7−/− APC. CD8 cells from CD28+/+ or CD28 −/− 2C mice were treated with SIY peptide in the presence of irradiated B7h+/+ or B7h−/− APC. After 4 days, T cells were restimulated with different concentrations of plate-bound anti-CD3. Proliferation was assayed 24 h after restimulation by adding [3H]thymidine to the culture for the last 8 h. (F) Eff and Tol CD8 cells were generated as described in Figure 1F. These cells were restimulated with plate-bound anti-CD3 for 4 h. Nuclear extracts prepared from nonrestimulated and restimulated cells were probed with ERK, NFATc1 and NFκB p65 antibodies. H3 was used as loading control. Relative Western blotting signals were inducted. (G) Eff and Tol CD8 cells were restimulated with anti-CD3 for 30 min and cytoplasmic fractions were probed with PKCθ and PLCγ1 antibodies and phosphospecific antibodies against PLCγ1 and ERK. β-Actin was used as loading control. Relative Western blotting signals were inducted. (H) Grail, cbl-b, Itch and HPRT mRNA expression in restimulated effector and tolerant CD8 T cells (as in F) was analyzed by RT–PCR.
