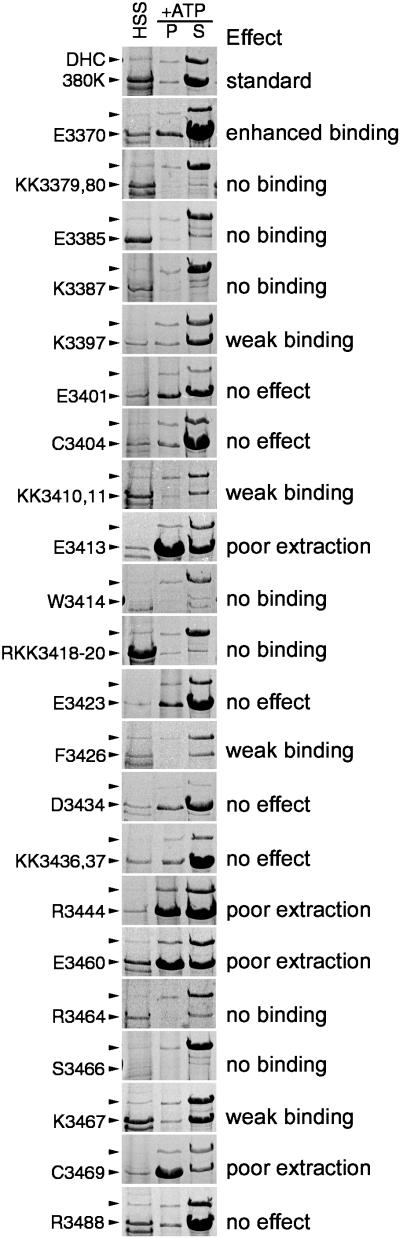
Figure 4.
Each block shows a portion of three Coomassie blue-stained gel lanes representing the HSS and the microtubule pellet (P) and supernatant (S) after ATP extraction. The top (380K) panel shows the relative binding of the control, unaltered head domain fragment; the remaining panels are representative of the amino acid substitution listed on the left. Positions of both the native DHC and the 380-kDa head domain fragment are marked with arrowheads. Although the levels of polypeptide overexpression appear to vary between the substitutions, they are generally consistent among clones isolated for each transformation and remain stable over time. In comparing the affinity, the relative amount of the 380 kDa fragment to the native DHC is important. For example, in clone KK3379,80, the altered 380-kDa polypeptide is much more abundant in the HSS but is virtually absent in the ATP extract. Two substitutions showed reproducibly low levels of polypeptide expression (W3414 and S3466). Their pattern of binding was confirmed by immunoblotting.