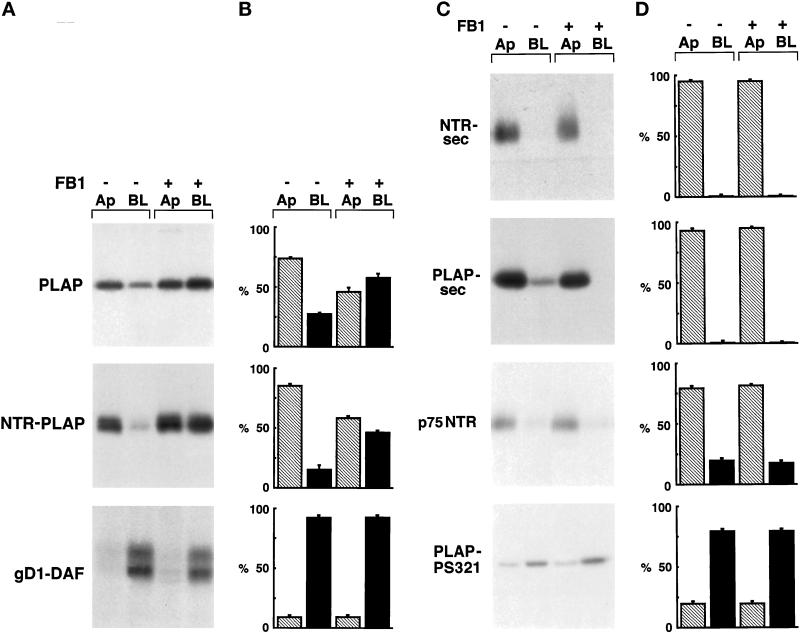
Figure 6.
Surface expression of GPI-anchored, secretory, and transmembrane proteins after FB1 treatment. (A) FRT cells stably expressing PLAP, NTR-PLAP, or gD1-DAF were grown to confluence in the absence (−) or presence (+) of FB1, as described in MATERIALS AND METHODS. After 30 min of labeling with [35S]met–cys or [35S]cys and a 2-h chase, cells were surface biotinylated from the apical (Ap) or the basolateral (BL) domain, lysed, immunoprecipitated against specific antibodies, and reprecipitated against streptavidin. (B) Amounts of labeled proteins were quantified from three independent experiments and are shown as means ± SD. Hatched bars represent apical proteins, and black bars represent basolateral proteins. Apical PLAP and NTR-PLAP, but not basolateral gD1-DAF, became unpolarized after FB1 treatment. (C) FRT cells expressing PLAP-sec, NTR-sec, p75NTR, or PLAP-PS321 were grown to confluence in the absence (−) or presence (+) of FB1, as described in MATERIALS AND METHODS. After labeling with [35S]met–cys or [35S]cys for 20 min and a chase of 2 h, apical and basolateral media were collected separately and cells were surface biotinylated from apical or basolateral domains. Polarized secretion of the secretory forms was revealed by immunoprecipitation in the apical and basolateral medium (top two panels), whereas biotinylated transmembrane proteins were revealed by double immunoprecipitation with specific antibody and streptavidin beads (bottom two panels). (D) Amounts of labeled proteins were quantified from three independent experiments and are shown as means ± SD. Hatched bars represent apical proteins, and black bars represent basolateral proteins. FB1 does not alter the sorting of transmembrane and secretory proteins.