Abstract
In 2005, the Clinical and Laboratory Standards Institute published MIC interpretive criteria for 13 antimicrobial agents used for either therapy or prophylaxis of Neisseria meningitidis infections. The MIC method includes the use of lysed horse blood-supplemented Mueller-Hinton broth with incubation in 5% CO2 for 20 to 24 h. Since some clinical laboratories might prefer the option of disk diffusion testing for infrequently encountered isolates a multicenter collaborative study was conducted to evaluate the reproducibility of a disk diffusion method for testing isolates of N. meningitidis. Interpretive criteria were developed for 12 antimicrobial agents. Four laboratories tested a common collection of 50 meningococcal strains and then tested 25 unique isolates per laboratory. Isolates were tested using Mueller-Hinton sheep blood agar plates incubated for 20 to 24 h in 5% CO2; they were also tested by the reference broth microdilution method in parallel. Pooling of the MIC and disk diffusion data from the common and unique isolates provided a sufficient sample size to develop susceptible, intermediate, and resistant zone diameter interpretive criteria using the error rate-bounded method for the following agents: chloramphenicol, trimethoprim-sulfamethoxazole, ciprofloxacin, and rifampin. Due to the lack of resistant strains at the present time, “susceptible only” interpretive criteria were proposed for cefotaxime, ceftriaxone, meropenem, azithromycin, and minocycline. The numbers of minor interpretive errors with penicillin and ampicillin disk tests were unacceptably high and precluded recommended testing of those agents by the disk method. However, amdinocillin, an agent that preferentially binds to the altered penicillin binding protein responsible for diminished penicillin susceptibility, has potential utility as a surrogate screening reagent for ampicillin resistance. A disk diffusion breakpoint was derived for nalidixic acid to serve as a surrogate marker for gyrase A mutations associated with diminished fluoroquinolone susceptibility. Disk diffusion testing with meningococci can be performed in a reproducible manner with several antimicrobial agents and represents a practical and cost-effective option for testing sporadic clinical isolates or for surveillance purposes by resource-limited laboratories.
The Clinical and Laboratory Standards Institute (CLSI) published MIC interpretive criteria for 13 antimicrobial agents for Neisseria meningitidis for the first time in 2005 (11). The criteria apply to both the broth microdilution method using lysed horse blood-supplemented Mueller-Hinton broth and the agar dilution method using Mueller-Hinton agar supplemented with 5% sheep blood. Both broth and agar MIC tests must include incubation in 5% CO2 to achieve consistent and accurate results (18). The 13 antimicrobial agents for which interpretive criteria are available include those used for both therapy and prophylaxis of invasive meningococcal infections. The goal for developing MIC breakpoints was to facilitate surveillance of emerging resistance in N. meningitidis strains and to assist in managing treatment of invasive disease and prophylaxis of case contacts. The interpretive criteria were developed based upon MIC distributions of wild-type strains, MICs of strains with genetically-characterized resistance mechanisms, limited published clinical data, and the use of pharmacokinetic and pharmacodynamic simulations, as outlined in CLSI publication M23-A2 (8). Prior to the development of these standard CLSI MIC methods and breakpoints, studies using different susceptibility testing approaches showed discordant estimates of the frequency of meningococcal strains with decreased susceptibility to penicillin in the United States. Diminished penicillin susceptibility has been shown previously to result from production of altered penicillin binding protein 2 (PBP2) (2, 24). Two studies conducted by the Centers for Disease Control and Prevention (CDC) using a reference broth microdilution method found only 3% of strains with elevated penicillin MICs of 0.12 μg/ml (17, 23). However, another U.S. surveillance study that employed a commercial MIC test method reported 30% of strains with elevated penicillin MICs of 0.09 to 0.25 μg/ml (22). Applying the newly approved CLSI breakpoints to 325 CDC Active Bacterial Core surveillance isolates recovered between 1997 and 2002 revealed that 13.2% of the isolates were not susceptible to penicillin (MIC ≥ 0.12 μg/ml) and 5.8% were not susceptible to ampicillin (MIC ≥ 0.25 μg/ml) and that 52.7% were resistant to sulfisoxazole (MIC ≥ 4 μg/ml), 55% to trimethoprim-sulfamethoxazole (MIC ≥ 0.25 μg/ml), and 1.5% to rifampin (MIC ≥ 1 μg/ml) (J. H. Jorgensen, S. A. Crawford, and N. E. Rosenstein, Abstr. 43rd Ann. Meet. Infect. Dis. Soc. Amer., abstr. 503, p. 130, 2005).
Clinical microbiology or public health laboratories may receive requests to perform susceptibility testing of isolates from an individual patient when the patient is not responding clinically, when a cluster of meningococcal cases occurs, or when an outbreak is recognized and appropriate prophylactic agents need to be identified to control the spread of infections. For example, the inability to recognize rifampin-resistant isolates early was responsible for the failure of prophylactic rifampin to prevent meningococcemia among close contacts of patients (16, 21). In such instances, laboratories have sometimes performed MIC determinations using a commercial gradient diffusion method (11, 20, 28). Previous studies of meningococcal susceptibility performed using the disk diffusion method have shown that standard content penicillin (10 U), ampicillin (10 μg), and oxacillin (1 μg) disks do not reliably discriminate between penicillin-susceptible and relatively penicillin-resistant strains (5, 6). However, there have been encouraging data regarding the use of low-content penicillin (2 U) and ampicillin (2 μg) disks to identify meningococci with decreased susceptibility to beta-lactam drugs (5, 6). Evaluation of the results of these previous studies, however, is complicated by the numerous differences in the testing methods, e.g., the different media used for MIC and disk diffusion tests, different inoculum densities and disk contents, and use of either ambient air or CO2 atmosphere for incubation (1, 3, 5, 6, 19). This has resulted in some sharp differences of opinion regarding the utility of disk diffusion testing for N. meningitidis (3; J. Campos, Letter, J. Clin. Microbiol. 37:879-880, 1999).
Initial studies to develop a disk diffusion method and interpretive criteria for meningococci were conducted using a diverse collection of meningococcal strains in a single laboratory, the University of Texas Health Science Center, San Antonio (UTHSCSA). The preliminary studies suggested that reproducible disk diffusion results could be obtained for a variety of antimicrobial agents by use of Mueller-Hinton sheep blood agar with incubation for 20 to 24 h at 35°C in a 5% CO2 atmosphere (J. H. Jorgensen, S. A. Crawford, and L. C. Fulcher, Abstr. 105th Gen. Meet. Amer. Soc. Microbiol., abstr. C-352, 2005). Those initial studies suggested the need to perform a multilaboratory study to assess the interlaboratory reproducibility of disk testing with meningococci and to derive potential disk diffusion breakpoints that might be accepted by the CLSI.
MATERIALS AND METHODS
Test isolates.
A collection of 50 meningococcal strains was assembled using both CDC Active Bacterial Core surveillance isolates from the United States and selected antimicrobial resistant isolates from outside the United States. Specifically there were 38 isolates from at least 10 U.S. states, 6 from Australia, 3 from Spain, and 1 each from Bangladesh, Canada, and Saudi Arabia. These included 3 isolates of serogroup A, 14 isolates of serogroup B, 20 isolates of serogroup C, 2 isolates of serogroup W135, 1 isolate of serogroup X, 9 isolates of serogroup Y, and 1 group Z strain. There were 21 strains with documented resistance to penicillin and 2 chloramphenicol-resistant, 2 quinolone-resistant, 7 rifampin-resistant, 24 sulfonamide-resistant, and 2 tetracycline-resistant strains, as described earlier (13, 15, 18). This collection was tested independently and in a blinded fashion by each of the four participating laboratories. In addition, each laboratory tested 25 unique isolates of meningococci from its own culture collection.
Biosafety precautions.
Since this study involved testing a large number of meningococcal isolates, and because laboratory-acquired infections have resulted in serious illness or death in the past (7), strict safety precautions were followed in this study. All aerosol-producing procedures and the reading of disk diffusion zones with plate lids removed were conducted within biosafety cabinets. All four laboratories required that each microbiologist performing the testing wear an impermeable laboratory coat and gloves during all procedures. One laboratory (National Institutes of Health) performed all testing under biosafety level 3 conditions. All microbiologists were immunized using the meningococcal quadrivalent polysaccharide vaccine.
Antimicrobial disks and their contents.
Disks of the following contents were purchased from BD Microbiology Systems (Cockeysville, MD) for use in three of the laboratories and from Remel (Lenexa, KS) for one laboratory (CDC). These included penicillin (10 U), ampicillin (10 μg), cefotaxime (30 μg), ceftriaxone (30 μg), amdinocillin (10 μg), meropenem (10 μg), chloramphenicol (30 μg), rifampin (5 μg), trimethoprim-sulfamethoxazole (1.25 μg-23.5 μg), tetracycline (5 μg), minocycline (30 μg), ciprofloxacin (5 μg), nalidixic acid (30 μg), and azithromycin (15 μg).
Disk diffusion susceptibility tests.
The CLSI disk diffusion procedure (10) included use of 150-mm-diameter Mueller-Hinton agar supplemented with 5% defibrinated sheep blood (BD in three laboratories, Remel in the fourth [CDC]). Test inocula were prepared from meningococcal colonies grown on chocolate agar plates that had been incubated for 20 to 24 h in 5% CO2. Colonies were suspended in 0.9% saline solution to obtain a suspension equivalent to the turbidity of a 0.5 McFarland standard. The suspension was used to swab the surface of the agar plates prior to application of four disks per 150-mm plate (only four disks were used because large zone diameters were anticipated). Plates were incubated in a 5% CO2 atmosphere for 20 to 24 h prior to measurement of the zones of inhibition. In two laboratories, duplicate disk tests were incubated in a standard incubator that provided a 5% CO2 atmosphere and in candle extinction jars for provision of a CO2 atmosphere. Zones were measured from the top of the agar with the lids removed by use of reflected light. Some laboratories used calipers for zone diameter measurements, and some used simple rulers.
Broth microdilution MIC susceptibility tests.
MICs of each agent were determined using a single lot of frozen broth microdilution panels prepared in one laboratory (UTHSCSA) using the procedure described by CLSI (9). This included use of cation-adjusted Mueller-Hinton broth (Difco formulation; BD) supplemented with 3% lysed horse blood as the test medium. An inoculum suspension equivalent to the 0.5 McFarland standard was further diluted to provide a final inoculum density of 5 × 105 CFU/ml in the wells of the microdilution panels. Colony counts of the 0.5 McFarland standard suspension and positive-control wells of the microdilution panels were performed to ensure the desired inoculum concentrations. Panels were incubated in a 5% CO2 atmosphere for 20 to 24 h prior to visual determination of MICs.
Quality control strains.
For quality control of both the broth microdilution and disk diffusion tests, Streptococcus pneumoniae ATCC 49619 was employed for all drugs for which there are approved CLSI control ranges (11). Escherichia coli ATCC 25922 was used for quality control of ciprofloxacin, amdinocillin, minocycline, and nalidixic acid, which lack approved MIC control limits for the pneumococcal control strain (11).
Comparison of MICs and zone diameters.
MIC versus zone diameter scatterplots were prepared for the 50 challenge strains tested in all four laboratories and for the 100 unique meningococcal isolates tested in the four laboratories. Zone diameter interpretive criteria for each agent were derived using the modified error rate-bounded method (4).
RESULTS
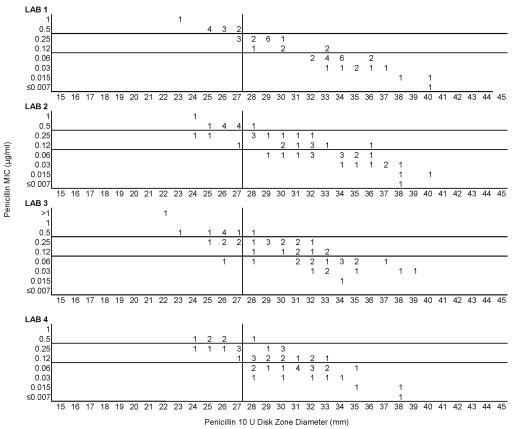
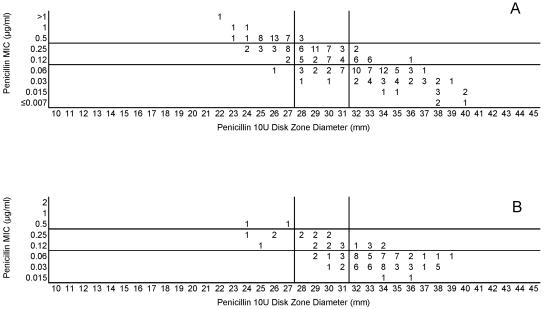
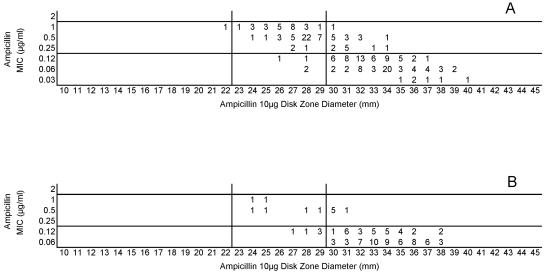
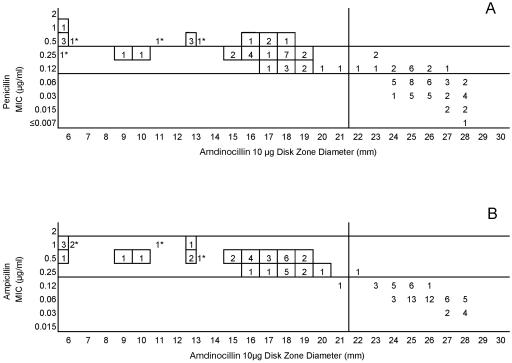
Testing of the 50 common isolates in the four laboratories resulted in very similar MIC and zone diameter determinations by each laboratory. There were no significant differences in zone diameters based upon the brand of Mueller-Hinton sheep blood agar or the brand of antimicrobial agent disks (data not depicted). However, zone diameters differed markedly when disk tests were incubated in candle extinction jars instead of a standard CO2 incubator. Zones were much larger and growth was somewhat poorer in the candle jars (data not depicted further). Figure 1 illustrates the similarity in MIC and zone diameter distributions with penicillin tested in the four laboratories. Similar results were obtained with the 50-strain collection with the remaining drugs in this study (data not depicted). Figure 2A depicts the same data included in Fig. 1, although all data points generated by the four laboratories are depicted on a single scattergram (n = 200). Figure 2B illustrates the MICs and zone diameters that resulted from testing penicillin with the unique 25 isolates tested separately by the four laboratories; i.e., all 100 data points are presented on a single scattergram. Best-fit interpretive criteria derived using the modified error rate-bounded method for penicillin are indicated as vertical lines in Fig. 2. Unfortunately, the proposed breakpoints resulted in a large number of minor errors (26%) with the zone diameter breakpoints selected (Table 1). Figure 3A depicts the combined test data from the four laboratories from tests of the challenge strains with ampicillin, while Fig. 3B indicates the pooled results of testing the unique isolates with ampicillin. While somewhat lower interpretive error rates were observed with ampicillin (as opposed to penicillin), error rates, especially minor errors, were still substantial (i.e., 11 to 13.5% and 19 to 26%; Table 1). Since the proposed interpretive criteria for both the penicillin and ampicillin disk tests showed unacceptably high minor error rates, a selected group of 102 strains of meningococci was tested using amdinocillin disks in one laboratory (UTHSCSA) as a potential surrogate marker for decreased susceptibility to beta-lactam agents due to production of a modified PBP2. Figure 4 illustrates the potential utility of amdinocillin as a disk screening test. The amdinocillin disk results appear to differentiate effectively between ampicillin-susceptible isolates and those isolates with decreased susceptibility to ampicillin due to PBP2 alterations. (Fig. 4B). However, amdinocillin zones correlated less well with penicillin MICs (Fig. 4A). There was only one major error (0.9%) with the proposed amdinocillin breakpoint compared with ampicillin MIC results, although there were 13.7% minor errors compared with penicillin MICs.
FIG. 1.
Comparison of penicillin MICs and disk zone diameters recorded by the four laboratories with the 50-strain collection tested in common.
FIG. 2.
(A) Combined penicillin MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Penicillin MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
TABLE 1.
Interpretive category errors associated with application of the proposed breakpoints to the strains tested in this study
| Antimicrobial agent (disk content in μg unless otherwise noted) | % Error for 50 strains tested in four laboratoriesa
|
% Error for 100 unique strainsa
|
||||
|---|---|---|---|---|---|---|
| m | M | VM | m | M | VM | |
| Penicillin (10 U) | 26 | 0.5 | 0 | 19 | 0 | 0 |
| Ampicillin (10) | 13.5 | 0 | 0 | 11 | 0 | 0 |
| Cefotaxime (30) | 0 | 0 | 0 | 0 | 0 | 0 |
| Ceftriaxone (30) | 0 | 0.5 | 0 | 0 | 0 | 0 |
| Meropenem (10) | 0 | 0 | 0 | 0 | 0 | 0 |
| Chloramphenicol (30) | 1 | 0 | 0 | 2 | 0 | 0 |
| Rifampin (5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Trimethoprim-sulfamethoxazole (1.25-23.75) | 0 | 0 | 0 | 2 | 0 | 0 |
| Minocycline (30) | 0 | 0 | 0 | 0 | 0 | 0 |
| Azithromycin (15) | 0 | 0 | 0 | 0 | 0 | 1 |
| Ciprofloxacin (5) | 1 | 0 | 0 | 0 | 0 | 0 |
| Nalidixic acid (30) | 0 | 0 | 0 | 0 | 0 | 0 |
m, minor interpretive error (i.e., intermediate category versus susceptible or resistant categories); M, major interpretive error, susceptible by the reference MIC method, resistant by the disk test; VM, very major interpretive error, resistant by the reference MIC method, susceptible by the disk test.
FIG. 3.
(A) Combined ampicillin MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Ampicillin MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 4.
(A) Penicillin MICs and amdinocillin disk zone diameters recorded with 102 selected strains tested in one laboratory (UTHSCSA). (B) Ampicillin MICs and amdinocillin disk zone diameters recorded with 102 selected strains tested in one laboratory (UTHSCSA). The vertical line represents the single proposed zone diameter breakpoint for screening purposes. Strains with known altered PBP2 are indicated in boxes. Strains that were not examined for PBP2 alterations are indicated by asterisks.
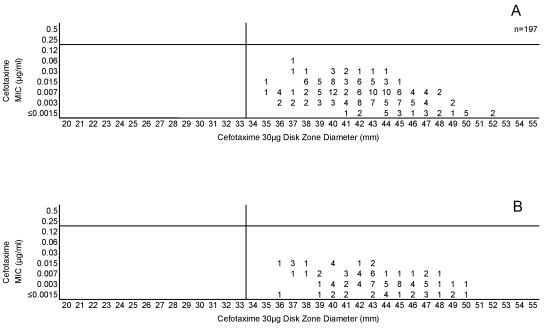
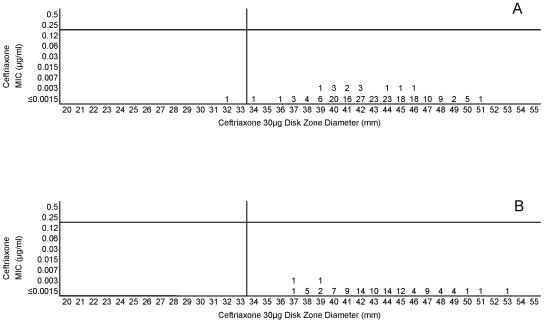
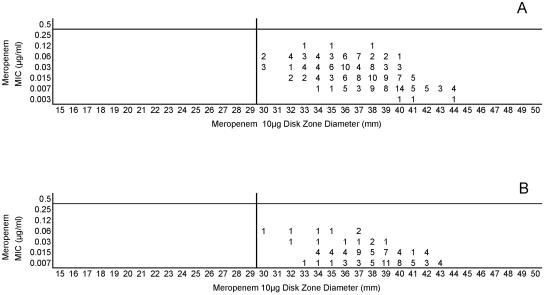
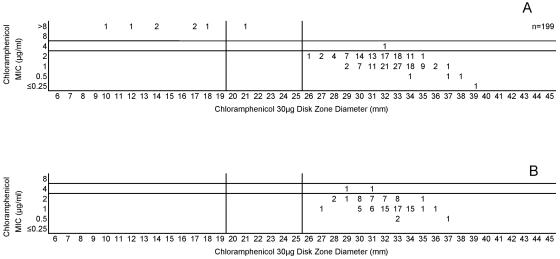
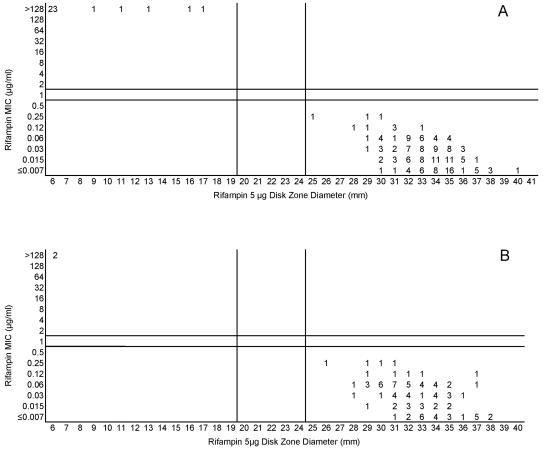
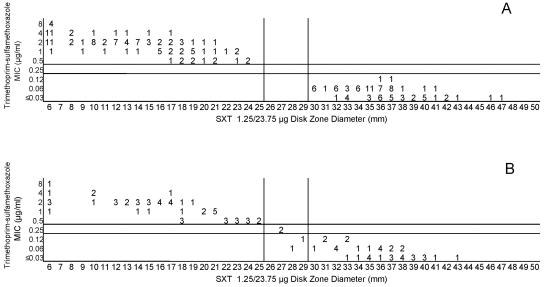
Cefotaxime, ceftriaxone, and meropenem were highly active against all meningococci examined in this study. Figures 5A and B, 6A and B, and 7A and B, respectively, depict the MIC zone size correlations with these three drugs observed when testing the two organism collections in this study. For all three of these agents, a single “susceptible only” zone diameter breakpoint is indicated on each scattergram. Figures 8, 9, and 10 represent MIC zone diameter comparisons for chloramphenicol, rifampin, and trimethoprim-sulfamethoxazole, respectively. The availability of strains with resistance mechanisms affecting the activities of those agents allowed proposed interpretive criteria for susceptible, intermediate, and resistant categories with few interpretive errors (Table 1).
FIG. 5.
(A) Combined cefotaxime MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Cefotaxime MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 6.
(A) Combined ceftriaxone MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Ceftriaxone MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 7.
(A) Combined meropenem MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Meropenem MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 8.
(A) Combined chloramphenicol MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Chloramphenicol MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 9.
(A) Combined rifampin MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Rifampin MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 10.
(A) Combined trimethoprim-sulfamethoxazole MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. Trimethoprim-sulfamethoxazole disks are abbreviated as SXT on these graphs. (B) Trimethoprim-sulfamethoxazole MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
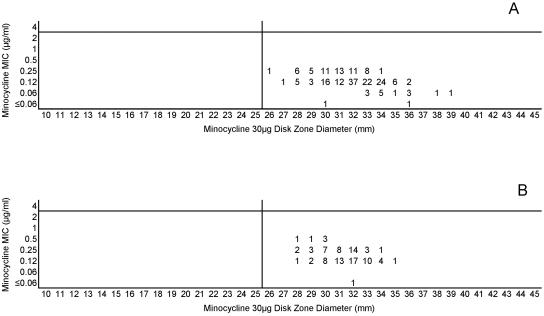
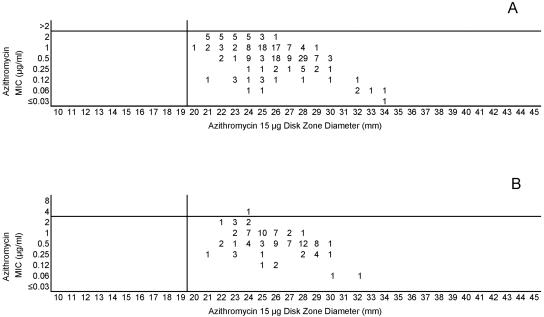
The lack of strains with acquired resistance mechanisms that affected minocycline and azithromycin precludes defining breakpoints other than the susceptibility breakpoint. Figures 11A and B and 12A and B indicate the single disk diffusion breakpoints proposed for those two agents. Last, only two strains were available for inclusion in this study that demonstrated reduced fluoroquinolone susceptibility. The high potency of ciprofloxacin against meningococci results in only modest elevations of MICs and concomitant reductions in zone diameters in strains that contain gyrA mutations, i.e., the principle target of ciprofloxacin in meningococci. However, the combined data set allows interpretive criteria to be established, as shown in Fig. 13. The nonfluorinated quinolone nalidixic acid appears to be a useful indicator of gyrA mutations in meningococcal testing. Figure 14 demonstrates that strains with reduced quinolone susceptibility can be readily separated from normal wild-type meningococci by use of a single-zone-diameter breakpoint.
FIG. 11.
(A) Combined minocycline MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Minocycline MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 12.
(A) Combined azithromycin MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Azithromycin MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 13.
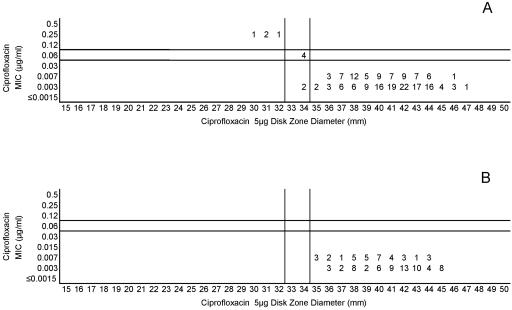
(A) Combined ciprofloxacin MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Ciprofloxacin MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical lines represent the proposed zone diameter breakpoints.
FIG. 14.
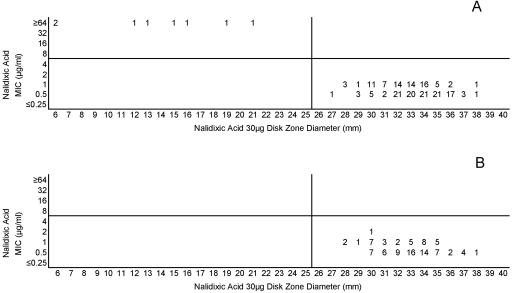
(A) Combined nalidixic acid MICs and disk zone diameters recorded with the 50-strain collection by the four laboratories. (B) Nalidixic acid MICs and disk zone diameters recorded with 100 unique meningococcal isolates contributed individually by the four laboratories. The vertical line represents the proposed single-zone-diameter breakpoint for screening purposes.
Both the approved MIC interpretive criteria and the zone diameter interpretive criteria derived in this study are summarized in Table 2. The proposed breakpoints are associated with generally low numbers of category-interpretive errors, with the notable exceptions of those of penicillin and ampicillin (Table 1).
TABLE 2.
Disk diffusion interpretive criteria for Neisseria meningitidis on the basis of the four-laboratory study
| Antimicrobial agent (disk content in μg unless otherwise noted) | MIC (μg/ml)
|
Zone diam (mm)
|
||||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| Ampicillin (10) | ≤0.12 | 0.25-1 | ≥2 | ≥30 | 23-29 | ≤22 |
| Penicillin (10 U) | ≤0.06 | 0.12-0.25 | ≥0.5 | ≥32 | 27-31 | ≤26 |
| Amdinocillina (10) | ≤0.12 | ≥0.25 | ≥23 | ≤22 | ||
| Cefotaxime (30) | ≤0.12 | ≥34 | ||||
| Ceftriaxone (30) | ≤0.12 | ≥34 | ||||
| Meropenem (10) | ≤0.25 | ≥30 | ||||
| Chloramphenicol (30) | ≤2 | 4 | ≥8 | ≥26 | 20-25 | ≤19 |
| Rifampin (5) | ≤0.5 | 1 | ≥2 | ≥25 | 20-24 | ≤19 |
| Trimethoprim-sulfamethoxazole (1.25-23.75)b | ≤0.12-2.3 | 0.25-4.75 | ≥0.5-9.5 | ≥30 | 26-29 | ≤25 |
| Minocycline (30) | ≤2 | ≥26 | ||||
| Azithromycin (15) | ≤2 | ≥20 | ||||
| Ciprofloxacin (5) | ≤0.03 | 0.06 | ≥0.12 | ≥35 | 33-34 | ≤32 |
| Nalidixic acid (30) | ≤4 | ≥8 | ≥26 | ≤25 | ||
Amdinocillin disks can be used to screen for nonsusceptibility to ampicillin (MIC > 0.25 μg/ml).
Trimethoprim-sulfamethoxazole is the preferred disk for detection of sulfonamide resistance.
DISCUSSION
The primary goal of this study was to determine whether disk diffusion was a reliable method for assessing the susceptibility of N. meningitidis to a variety of therapeutic and prophylactic agents and, if so, to develop interpretive criteria for those antimicrobial agents. A previous study (Jorgensen et al., Abstr. 105th Gen. Meet. Amer. Soc. Microbiol.) demonstrated that disk diffusion zones can be measured reproducibly using the test conditions described in this study. In that single laboratory study, 94.9% zones measured by four separate observers agreed within 3 mm for the 14 antimicrobial agents studied. In the present study, all four laboratories achieved similar disk diffusion results when they tested a collection of 50 meningococcal strains that included a series of contemporary wild-type strains and strains exhibiting a variety of resistance mechanisms. There was very good agreement among both the MIC results and the zone diameters reported by the participating laboratories for the 12 antimicrobial agents included in this study. The favorable agreement among the laboratories and the availability of strains with resistance or decreased susceptibility to several of the antimicrobial agents has led to the proposed interpretive criteria that define standard susceptible, intermediate, or resistant categories or categories for antimicrobial agents for which there currently is no recognized resistance (the “susceptible only” category).
For most of the antimicrobial agents included in this investigation, the interpretive error rates (very major, major, and minor) are low and are similar to those observed for other fastidious and nonfastidious bacterial isolates. This is encouraging given the large zone diameters produced by many of the drugs when meningococci are tested. It is disappointing, however, that there were an excessive number of minor interpretive errors when the standard content penicillin (10 U) and ampicillin (10 μg) disks were used in this study. An earlier study (Jorgensen et al., Abstr. 105th Gen. Meet. Amer. Soc. Microbiol.) was not able to achieve lower error rates by using lower-content penicillin (1 U) or ampicillin (2 μg) disks. Thus, we explored a variety of other beta-lactam agents as an alternate means of identifying isolates with reduced susceptibility to penicillin and ampicillin. Other disks examined in one laboratory (UTHSCSA) included amoxicillin-clavulanic acid, cefoxitin, ticarcillin, and amdinocillin. Of those, only amdinocillin showed promise as a screening test for isolates with decreased penicillin and ampicillin susceptibility. The fact that amdinocillin specifically binds to PBP2 (numbered according to the E. coli numbering system) likely explains why meningococci with mosaic PBP2 (26) can be detected effectively with an amdinocillin disk test. The results of the amdinocillin disk test correlate better with elevated ampicillin MICs than with elevated penicillin MICs. An earlier study demonstrated a closer correlation between elevated ampicillin MICs and the presence of PBP2 alterations than the correlation of penicillin MICs with the altered drug target (18). Disk diffusion testing with amdinocillin may be a cost-effective approach to screening a large number of isolates that have reduced susceptibility to beta-lactams, particularly when the isolates are suspected to belong to a single or a few clonal groups (27).
Similarly, the nonfluorinated quinolone nalidixic acid can be used to screen for isolates with gyrA mutations that decrease the activity of fluoroquinolones. Because meningococcal strains with decreased fluoroquinolone susceptibility have been reported in several different parts of the world (B. Alcala, C. Salcedo, L. de la Fuente, L. Arreaza, M. J. Urfa, R. Abad, R. Enriquez, J. A. Velazquez, M. Motge, and J. de Batlle, Letter, J. Anitimicrob. Chemother. 53:409, 2004; A. Corso, D. Faccone, M. Miranda, M. Rodriguez, M. Regueira, C. Carranza, C. Vencina, J. A. Vazquez, and M. Galas, Letter, J. Antimicrob. Chemother. 55:596-597, 2005; and T. R. Schultz, J. W. Tapsall, and P. A. White, Letter, Antimicrob. Agents Chemother. 44:1116, 2000) and have the potential for development of higher level fluoroquinolone resistance (25), screening with nalidixic acid by use of either MIC or disk could be a useful epidemiologic and diagnostic tool.
The disk diffusion method and interpretive criteria described herein provide a convenient method that can be used for epidemiologic surveys of emerging meningococcal resistance, or for clinical situations in which a physician needs confirmation that the drugs normally used for empirical therapy or prophylaxis of invasive meningococcal infections will likely be effective, or in resource-limited settings in which MIC determination methods are not readily available. It is important to follow the methodological details outlined above and in the CLSI document in order to obtain reproducible disk diffusion test results. Incubation of tests in candle extinction jars is not recommended as a means to achieve a suitable CO2 atmosphere. We do not recommend that disk testing be used to assess the activity of penicillin in cases of meningitis; in those situations, a MIC determination would be preferred.
The CLSI Antimicrobial Susceptibility Testing Subcommittee has reviewed the data presented in Table 2 and incorporated the disk diffusion breakpoints in CLSI publication M100-S16 (12), with the exception of the penicillin, ampicillin, and amdinocillin breakpoints. The CLSI concluded that the rates of minor errors with penicillin and ampicillin disk tests were too high for those tests to be recommended. The CLSI has not yet considered the possibility of using amdinocillin as a surrogate disk to screen for diminished penicillin and ampicillin susceptibility. The latest CLSI publication (12) now includes interpretive criteria for both MIC and disk diffusion testing of the drugs most often used for therapy and prophylaxis of meningococcal disease.
Acknowledgments
This study was supported in part by grant RS1/CCR622402 from the Centers for Disease Control and Prevention. Most of the U.S. isolates in this study were collected as part of the Active Bacterial Core surveillance (ABCs) effort of the Emerging Infections Program of the CDC. The Meningitis and Special Pathogens Laboratory of the CDC facilitated access to those strains. Resistant non-U.S. isolates were generously provided by John Turnidge from Adelaide, Australia, and two chloramphenicol-resistant isolates and a strain with diminished quinolone susceptibility were kindly provided by Professor John Tapsall from Randwick, NSW, Australia. Julio Vazquez (Spain) kindly provided a strain with diminished fluoroquinolone susceptibility. Robert Rennie (Canada) provided eight isolates with elevated penicillin MICs.
REFERENCES
- 1.Antignac, A., M. Ducos-Galand, A. Guiyoule, R. Pires, J.-M. Alonso, and M.-K. Taha. 2003. Neisseria meningitidis strains isolated from invasive infections in France (1999-2002): phenotypes and antibiotic susceptibility patterns. Clin. Infect. Dis. 37:912-920. [DOI] [PubMed] [Google Scholar]
- 2.Antignac, A., P. Kriz, G. Tzanakaki, J.-M. Alonso, and M.-K. Taha. 2001. Polymorphism of Neisseria meningitidis penA gene associated with reduced susceptibility to penicillin. J. Antimicrob. Chemother. 47:285-296. [DOI] [PubMed] [Google Scholar]
- 3.Block, C., Y. Davidson, and N. Keller. 1998. Unreliability of disc diffusion test for screening for reduced penicillin susceptibility in Neisseria meningitidis. J. Clin. Microbiol. 36:3103-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunden, M. N., G. E. Zurenko, and B. Kapik. 1992. Modification of the error-bounded classification scheme for use with two MIC breakpoints. Diagn. Microbiol. Infect. Dis. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 5.Campos, J., G. Trujillo, T. Seuba, and A. Rodgriguez. 1992. Discriminative criteria for Neisseria meningitidis isolates that are moderately susceptible to penicillin and ampicillin. Antimicrob. Agents Chemother. 36:1028-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos, J., P. M. Mendelman, M. U. Sako, D. O. Chaffin, A. L. Smith, and J. A. Sáenz-Nieto. 1987. Detection of relatively penicillin G-resistant Neisseria meningitidis by disk susceptibility testing. Antimicrob. Agents Chemother. 31:1478-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 2002. Laboratory-acquired meningococcal disease—United States, 2000. Morb. Mortal. Wkly. Rep. 51:141-144. [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute/NCCLS. 2001. Development of in vitro susceptibility testing criteria and quality control parameters. Approved guideline M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Clinical and Laboratory Standards Institute/NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Clinical and Laboratory Standards Institute/NCCLS. 2003. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A8. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11.Clinical and Laboratory Standards Institute/NCCLS. 2005. Performance standards for antimicrobial susceptibility testing. Supplement M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 12.Clinical and Laboratory Standards Institute/NCCLS. 2006. Performance standards for antimicrobial susceptibility testing. Supplement M100-S16. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 13.Crawford, S. A., K. R. Fiebelkorn, J. E. Patterson, and J. H. Jorgensen. 2005. International clone of Neisseria meningitidis serogroup A with tetracycline resistance due to tet(B). Antimicrob. Agents Chemother. 49:1198-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahar, O., H. A. Lopardo, and E. A. Rubeglio. 2002. Value of Etest penicillin V and penicillin G strips for penicillin susceptibility testing of Neisseria meningitidis. Diagn. Microbiol. Infect. Dis. 43:119-121. [DOI] [PubMed] [Google Scholar]
- 15.Fiebelkorn, K. R., S. A. Crawford, and J. H. Jorgensen. 2005. Mutations in folP associated with elevated sulfonamide MICs in Neisseria meningitidis clinical isolates from five continents. Antimicrob. Agents Chemother. 49:536-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser, A., A. Gafter-Gvili, and P. Leibovici. 2005. Prophylactic use of antibiotics for prevention of meningococcal infections: systematic review and meta-analysis of randomized trials. Eur. J. Clin. Microbiol. Infect. Dis. 24:172-181. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, L. A., F. C. Tenover, C. Baker, B. D. Plikaytis, M. W. Reeves, S. A. Stocker, R. E. Weaver, and J. D. Wenger. 1994. Prevalence of Neisseria meningitidis relatively resistant to penicillin in the United States, 1991. J. Infect. Dis. 169:438-441. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen, J. H., S. A. Crawford, and K. R. Fiebelkorn. 2005. Susceptibility of Neisseria meningitidis to sixteen antimicrobial agents and characterization of resistance mechanisms affecting some agents. J. Clin. Microbiol. 43:3162-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolas, P., J. D. Cavallo, R. Fabre, and G. Martet. 1998. Standardization of the Neisseria meningitidis antibiogram. Detection of strains relatively resistant to penicillin. Bull. W.H.O. 76:393-400. [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual, A., P. Joyanes, L. Martínez-Martínez, I. Suárez, and E. J. Perea. 1996. Comparison of broth microdilution and E-test for susceptibility testing of Neisseria meningitidis. J. Clin. Microbiol. 34:588-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainbow, J., E. Cebelinski, J. Bartkus, A. Glennen, D. Boxrud, and R. Lynfield. 2005. Rifampin-resistant meningococcal disease. Emerg. Infect. Dis. 11:977-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter, S. S., K. A. Gordon, P. R. Rhomberg, M. A. Pfaller, and R. N. Jones. 2001. Neisseria meningitidis with decreased susceptibility to penicillin: report from the SENTRY antimicrobial surveillance program, North America, 1998-99. Diagn. Microbiol. Infect. Dis. 41:83-88. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstein, N. E., S. A. Stocker, T. Popovic, F. C. Tenover, and B. A. Perkins. 2000. Antimicrobial resistance of Neisseria meningitidis in the United States, 1997. Clin. Infect. Dis. 30:212-213. [DOI] [PubMed] [Google Scholar]
- 24.Saez-Nieto, J. A., R. Lujan, S. Berron, J. Campos, M. Vinas, C. Fuste, J. A. Vazquez, Q.-Y. Zhang, L. D. Bowler, J. V. Martinez-Suarez, and B. G. Spratt. 1992. Epidemiology and molecular basis of penicillin-resistant Neisseria meningitidis in Spain: a 5-year history (1985-1989). Clin. Infect. Dis. 14:394-402. [DOI] [PubMed] [Google Scholar]
- 25.Schultz, T. R., P. A. White, and J. W. Tapsall. 2005. In vitro assessment of the further potential for development of fluoroquinolone resistance in Neisseria meningitidis. Antimicrob. Agents Chemother. 49:1753-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spratt, B. G. 1977. The mechanism of action of mecillinam. J. Antimicrob. Chemother. 3(Suppl. B):13-19. [DOI] [PubMed] [Google Scholar]
- 27.Stefanelli, P., C. Fazio, A. Neri, T. Sofia, and P. Mastrantonio. 2004. Emergence in Italy of a Neisseria meningitidis clone with decreased susceptibility to penicillin. Antimicrob. Agents Chemother. 48:3103-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez, J. A., L. Arreaza, C. Block, I. Ehrland, S. J. Gray, S. Heuberger, S. Hoffmann, P. Kriz, P. Nicolas, P. Olcen, A. Skoczynska, L. Spanjaard, P. Stefanelli, M.-K. Taha, and G. Tzanakaki. 2003. Interlaboratory comparison of agar dilution and Etest methods for determining the MICs of antibiotics used in management of Neisseria meningitidis infections. Antimicrob. Agents Chemother. 47:3430-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]