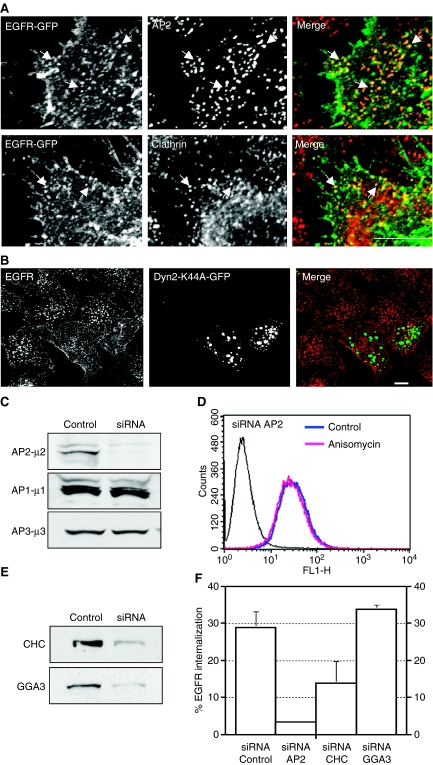
Figure 3. Anisomycin promotes endocytosis of epidermal growth factor receptor (EGFR) through clathrin-coated pits.
(A) HeLa cells expressing EGFR-green fluorescent protein (GFP) were treated with anisomycin (60 µm) for 8 min, fixed, stained with the indicated antibodies and analyzed by confocal microscopy. Bar represents 5 µm. (B) HeLa cells transfected with a plasmid encoding the GFP-tagged form of dynamin 2 K44A mutant (Dyn2-K44A-GFP) were incubated for 1 h on ice with a monoclonal antibody to the extracellular domain of EGFR, washed and allowed to internalize for 20 min at 37 °C in the presence of anisomycin (60 µm). Cells were then fixed, stained with a Cy3-conjugated donkey anti-mouse immunoglobulin G and analyzed by confocal microscopy. Bar represents 10 µm. (C) HeLa cells were transfected with small interfering RNA (siRNA) targeted to control (non-silencing) or to the µ2 subunit of the AP2 complex. Seventy-two hours after the second round of transfection, equivalent amounts of homogenate from control and AP2 siRNA-treated cells were subjected to SDS–PAGE and immunoblotted using an antibody to the µ subunit of AP2. To test the specificity of knockdown, lysates were also blotted to the µ subunits of AP1 and AP3. (D) Cells treated with AP2 siRNA were left untreated or stimulated with anisomycin for 20 min, and cell-surface EGFR was quantified by flow cytometry. (E) Western blot of total cellular levels of CHC or GGA3 in HeLa cells following transfection with either control (non-silencing) siRNA or the specific siRNA. (F) HeLa cells transfected with either non-targeting siRNA, siRNA-µ2, siRNA-CHC or siRNA-GGA3 were incubated with anisomycin for 20 min after which the amount of endogenous EGFR internalized was quantified by flow cytometry.