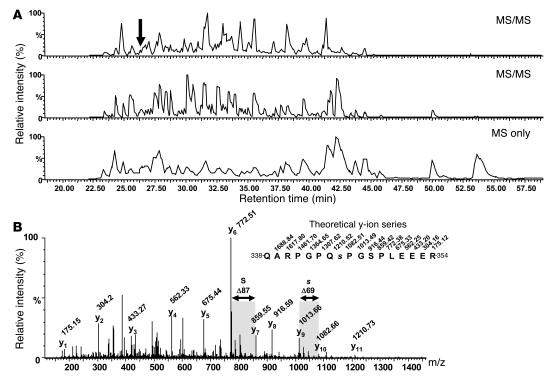
Figure 1. Mass spectrometry analysis of GM-CSF–induced p47phox phosphorylation: Ser345 was phosphorylated in primed human neutrophils.
(A) LC-MS/MS analysis of an aliquot of the p47phox tryptic peptide mixture (gradient: 0–38% acetonitrile in 35 minutes). The 2 upper panels depict the base peak chromatograms of electrospray ionization MS/MS experiments performed during the analysis in data-dependent acquisition mode. The lower panel represents the base peak chromatogram of the MS survey scans only. The arrow indicates the elution time of the phosphopeptide that was sequenced by MS/MS. (B) Identification of the phosphorylated peptide QARPGPQ[pS]PGSPLEEER (amino acids 338–354). The MS/MS spectrum displayed a near-complete y-ion series that reflects the amino acid sequence and the position of the phosphate group at Ser345. The elimination of phosphoric acid from the phosphoserine residue during MS/MS generated a dehydroalanine residue at the position corresponding to Ser345 (s; residue mass, 69 Da). The spectrum also demonstrated that the Ser348 residue (S) was not phosphorylated (residue mass, 87 Da).