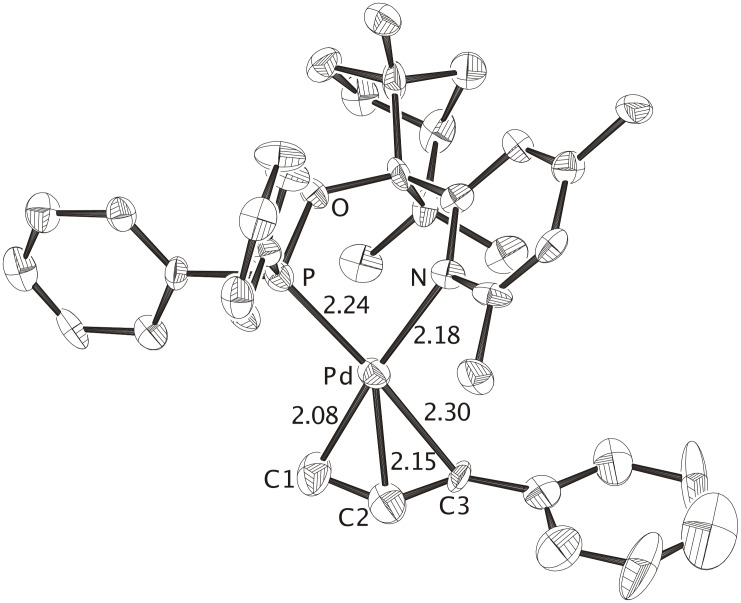
Figure 2.
X-ray crystal structure of the cationic complex Pd-FENOP-Me (CCDC 600369), the perchlorate counterion and hydrogen atoms are omitted. The allylic phenyl groups is positioned trans to phosphorus. In agreement with the "trans rule", C3-Pd is longer then C1-Pd. The nucleophile (i.e. malonate) is expected to attack at C3 yielding the branched product. Distances are given in Angstroms.