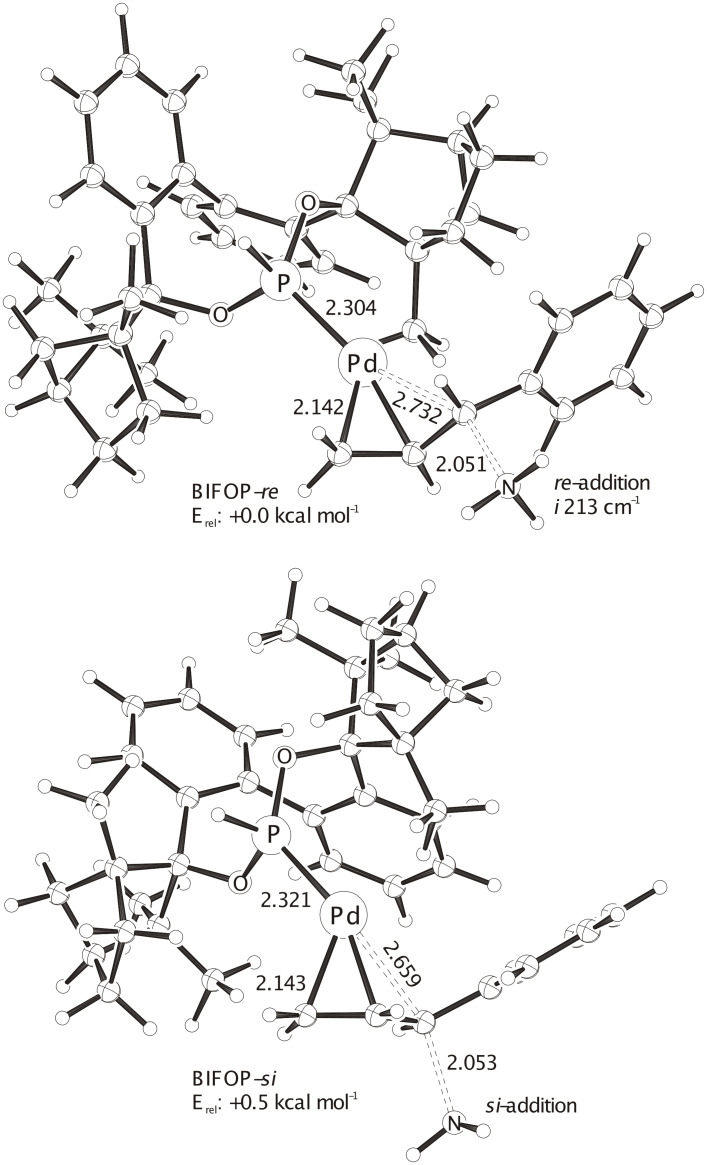
Figure 5.
The two most stable ONIOM(B3LYP/SDD(+ECP) (Pd) /6-31G* (C, H, O, N, P) : UFF) optimized transition structures with BIFOP-H, due to systematic conformational analysis (60° rotations at P-Pd). ZPE (unscaled) corrected total extrapolated energies: BIFOP-H-re: -1025.01553 H, BIFOP-H-si: -1025.01466 H. The by 0.5 kcal mol-1 slightly preferred re-addition of the NH3 model nucleophile corresponds to the experimental S-alkylation product.