Abstract
INTRODUCTION
Chronic open-angle glaucoma (COAG) is a leading cause of irreversible blindness worldwide, including in Canada. It presents a challenge in diagnosis, as disease often progresses without symptoms; an estimated 50% of cases are undetected.
SOURCES OF INFORMATION
MEDLINE searches, reference lists of articles, and expert knowledge from one of the authors (K.F.D.), a glaucoma specialist, were used.
MAIN MESSAGE
A casefinding approach using early referral to optometrists and ophthalmologists for early detection of COAG is helpful for patients with risk factors such as age above 50, a positive family history, black race, and myopia. Moderate evidence for referral also exists for the following risk factors: hypertension, type 2 diabetes mellitus, hypothyroidism, and sleep apnea. Treatment with intraocular pressure–lowering medication can arrest or slow the course of the disease, permitting patients to retain good visual function. Family physicians should be aware that some intraocular pressure–lowering medications, particularly topical beta-blockers, can pose iatrogenic harm to patients and result in or exacerbate such conditions as asthma, cardiovascular disturbances, depression, and sexual dysfunction.
CONCLUSION
Appropriate referral patterns and an understanding of common as well as serious side effects of glaucoma medications are important in optimizing management of patients at risk of developing, or who have, COAG.
Abstract
INTRODUCTION
Le glaucome chronique à angle ouvert (GCAO) est une cause majeure de cécité irréversible au Canada comme à l’échelle mondiale. Comme son évolution est souvent asymptomatique, il peut facilement passer inaperçu; on estime que 50% des cas demeurent non diagnostiqués.
SOURCE DE L’INFORMATION
On s’est servi de recherches dans MEDLINE, de listes bibliographiques d’articles et de l’expertise d’un des auteurs (K. F. D.), spécialiste du glaucome.
PRINCIPAL MESSAGE
Il y a avantage à demander une évaluation en optométrie ou en ophtalmologie pour les patients de plus de 50 ans, de race noire ou présentant d’autres facteurs de risque comme une histoire familiale positive ou une myopie. Certaines données indiquent également que l’hypertension, le diabète, l’hypothyroïdie et l’apnée du sommeil sont des facteurs de risque qui méritent un tel dépistage. On peut stopper ou ralentir le cours de la maladie à l’aide de médicaments qui abaissent la pression intra-oculaire, préservant ainsi la vision du patient. Le médecin de famille devrait savoir que certains de ces médicaments, notamment les bêta-bloqueurs topiques, peuvent avoir des effets iatrogéniques indésirables et exacerber des conditions comme l’asthme, les problèmes cardiovasculaires, la dépression et les dysfonction sexuelles.
CONCLUSION
La prise en charge optimale des patients qui ont ou qui risquent de développer un GCAO exige une bonne connaissance des effets indésirables sévères des médicaments anti-glaucome et un dépistage précoce par un spécialiste.
EDITOR’S KEY POINTS.
Glaucoma is an important cause of preventable blindness that is permanent. Half of patients with chronic open-angle glaucoma are not diagnosed due to its insidious onset, representing many cases of preventable blindness.
Major risk factors include age over 50, African heritage, positive family history, severe myopia, and raised intraocular pressure (IOP). Hypertension and type 2 diabetes also are associated.
Early treatment of elevated IOP permits most patients to preserve good visual function. Five classes of topical drops can be used to lower IOP; surgery is used for unresponsive cases to increase drainage of the anterior chamber.
Two of the drug classes, beta-blockers and carbonic anhydrase inhibitors, can cause serious systemic side effects. Steroid use by any route for as little as 2 or 3 weeks can increase IOP among susceptible patients.
POINTS DE REPÈRE DU RÉDACTEUR.
Le glaucome est une cause importante et évitable de cécité permanente. Parce que le glaucome chronique à angle ouvert débute de façon insidieuse, la moitié des cas ne sont pas diagnostiqués, ce qui représente plusieurs cas de cécité évitable.
Les principaux facteurs de risque sont l’âge (> 50), la descendance africaine, une histoire familiale positive, une forte myopie et une augmentation de la pression intra-oculaire (API). L’hypertension et le diabète de type II montrent aussi une association.
Un abaissement précoce de la PIO permet à la plupart des patients de conserver une bonne vision. Cinq classes de gouttes topiques peuvent être utilisées pour réduire la PIO; dans les cas réfractaires, une intervention permettra d’augmenter le drainage de la chambre antérieure.
Deux types de médicaments, les bloqueurs bêta et les inhibiteurs de l’anhydrase carbonique, peuvent avoir des effets indésirables systémiques graves. L’administration de stéroïdes par n’importe quelle voie et pour des périodes aussi courtes que 2 ou 3 semaines peut élever la PIO chez les sujets susceptibles.
Case 1
Mrs F., a 53-year-old African-Canadian woman, visits her family physician for a routine physical. She describes having trouble with driving as well as difficulty noticing steps or curbs. She has lost some peripheral vision, yet there is no history of trauma, ocular discomfort, or an obvious precipitating event. Ocular history is unremarkable. Medical history shows type 2 diabetes and mild asthma. Physical examination reveals blood pressure of 145/95 mm Hg, best corrected visual acuity of 20/25 in each eye, and a noticeable visual field defect with confrontational testing that is more pronounced in her right eye.
What are the risk factors for chronic open-angle glaucoma (COAG)? How can this condition be diagnosed?
Maximizing years of sight
Glaucoma is second only to age-related macular degeneration as a leading cause of irreversible blindness in North America.1 In patients of African descent, it is the most common cause of blindness with a higher prevalence, earlier age of onset, and greater severity of optic nerve damage than other conditions.2
Glaucoma is an intraocular pressure (IOP)–sensitive optic neuropathy that produces characteristic structural changes to the optic nerve head, often with correlating visual field defects. Among the types of glaucoma are COAG, secondary open-angle glaucoma, primary angle-closure glaucoma, secondary angle-closure glaucoma, congenital glaucoma, and juvenile glaucoma. The course of each of these disorders if left untreated is to progress inexorably to irreversible blindness. Chronic open-angle glaucoma is the most prevalent form of glaucoma in the Western world. It is underdiagnosed due to its lack of symptoms compared with acute or angle-closure glaucoma, which present with ocular pain, redness, blurred vision, and often nausea and vomiting.
Chronic open-angle glaucoma is difficult to identify because patients frequently have no symptoms at the time of diagnosis. Physiologic overlap in the visual fields between the eyes can hide early defects and thus delay detection until late in the disease when optic nerve damage puts central vision at risk.3 Fifty percent of glaucoma cases are undetected and remain untreated.2,4 These patients run a great risk of developing irreversible loss of vision.
Loss of vision is one of the most feared chronic disabilities.5 Glaucomatous visual field loss is correlated with a higher rate of automobile accidents. Vision-related quality of life is diminished due to limitations on social activities and dependence on others.6,7
As early diagnosis with appropriate management is the best way to maximize the number of years of sight for patients who have glaucoma, primary care physicians have a key role. Casefinding is more efficient and cost effective when targeting populations at particularly high risk of glaucoma, such as those older than 50, African-Canadians, and those with a family history of glaucoma. Also, those undergoing treatment for glaucoma could present to their general practitioners with potentially serious iatrogenic effects. Thus, a solid understanding of glaucoma and recognition of serious side effects associated with medical treatment is essential in family practice.
Sources of information
The information and approach of this article are derived from specific searches of MEDLINE, reference lists of articles, and the clinical experience of a glaucoma specialist (K.F.D.).
Anatomy of the eye and glaucomatous nerve changes
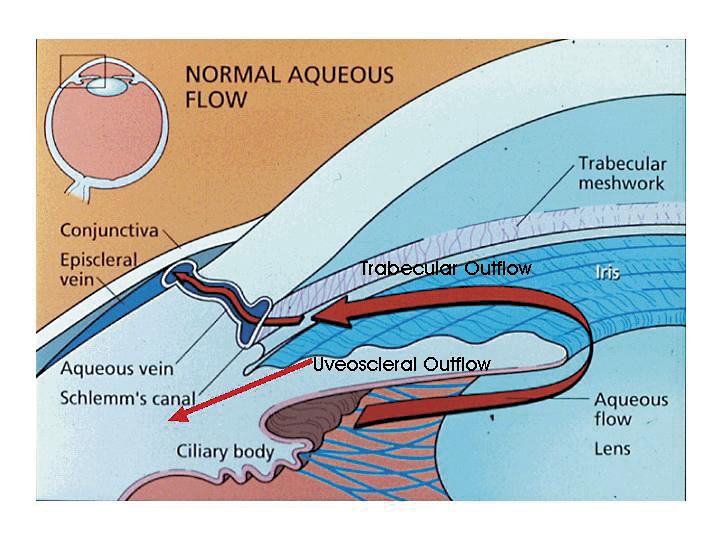
Aqueous humour provides nutrients for the avascular lens and corneal endothelium. It is produced by the ciliary body in the posterior chamber, circulates around the lens, passes through the pupil, and flows into the anterior chamber. Eighty percent of the outflow goes through the trabecular meshwork and drains into the episcleral venous system; the remaining 20% passes through the uveoscleral pathway via the interstitial spaces between the iris root and ciliary muscle (Figure 1).8,9 Chronic open-angle glaucoma is thought to be caused principally by an obstruction of the aqueous outflow deep within the trabecular meshwork, increasing IOP to a point where nerves are damaged.
Figure 1. Aqueous flow in the eye.
Aqueous humour provides nutrients for the avascular lens and corneal endothelium. It is produced by the ciliary body in the posterior chamber, circulates around the lens, through the pupil, and throughout the anterior chamber. Eighty percent of the outflow is through the trabecular meshwork and into the episcleral venous system; the remaining 20% drains out of the uveoscleral pathway via the interstitial spaces between the iris root and ciliary muscle. Chronic open-angle glaucoma is thought to be principally due to an obstruction of the aqueous outflow deep within the trabecular meshwork, causing an increase of ocular pressure to a point where nerve damage occurs.
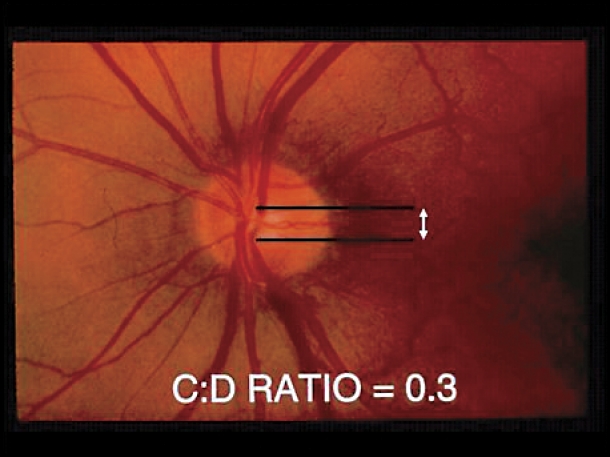
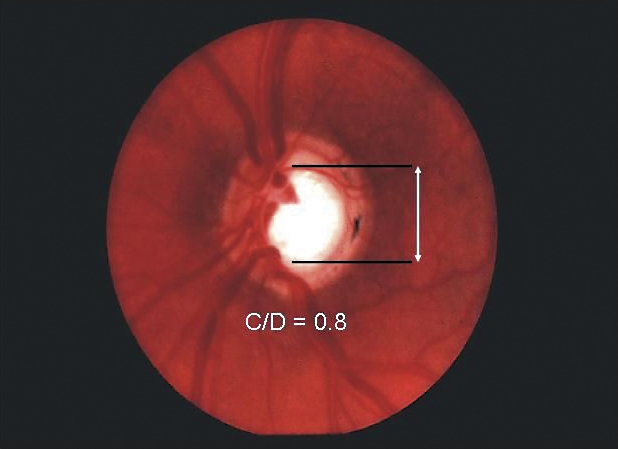
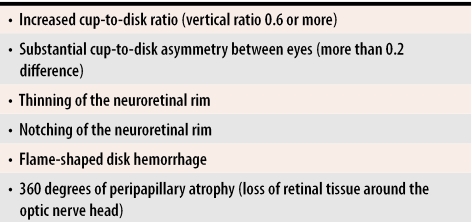
The optic nerve head consists of axons from approximately 1.2 million ganglion cells that have their cell bodies in the retina. Its blood supply derives from the ophthalmic artery, which supplies the anterior portion of the nerve through the central retinal artery, and the bulk of the prelaminar, laminar, and retrolaminar portions of the nerve via the short posterior ciliary arteries. Glaucomatous progression is best seen clinically by observing a characteristic three-dimensional excavation of the optic nerve head with slitlamp stereobiomicroscopy, or in two dimensions with direct ophthalmoscopy (in the case of the latter, a paler colour as well as blood vessel contour changes can help delineate the edge of the central cup). As axons within the nerve die (largely through apoptosis) and the plates of the lamina cribrosa sclerae (a specialized area of sclera) collapse due to IOP or ischemia, loss of optic nerve tissue produces a characteristic excavation or “cupping” of the optic nerve head. Diffuse enlargement is followed by elongation of the central cup of the optic disk to form a vertical oval. Thinning or notching of the disk rim, or disk hemorrhages might also be seen. When a vertical cup-to-disk ratio of 0.6 or greater is seen, glaucoma should be suspected (Figure 2). Often glaucoma affects the eyes asymmetrically with one cup appearing larger than the other; thus more than 0.2 asymmetry between the cup-to-disk ratios suggests loss of neural tissue and should also lead physicians to suspect glaucoma (Table 1).
Figure 2. Glaucomatous excavation of the optic nerve.
Loss of optic nerve tissue results in excavation or “cupping” of the optic nerve head, which is best viewed by direct ophthalmoscopy. A) Vertical cup-to-disk (C:D) ratio within the normal range. B) Glaucomatous cupping has a C:D ratio of 0.8. With a vertical C:D ratio 0.6 or greater, glaucoma should be suspected. Often glaucoma affects the eyes asymmetrically; one cup appears larger than the other. Thus >0.2 asymmetry between the C:D ratios of both eyes should also suggest glaucoma.
Table 1.
Glaucomatous changes of the optic nerve head viewed by ophthalmoscopy
Main message
Casefinding.
Strategies for identifying patients at risk for COAG include screening and casefinding. Screening involves identifying all individuals in a defined population who might benefit from further diagnostic assessment by an eye-care specialist. Casefinding is a term used for testing for a certain condition as the opportunity arises in the course of clinical care, such as during periodic health examinations.
Current strategies for mass screening are insufficiently accurate for detecting early COAG and have an unacceptably high rate of false-positive and false-negative results. Currently the Canadian Task Force on the Periodic Health Examination indicates that there is insufficient evidence to include or exclude ophthalmoscopy, tonometry, or automated perimetry to detect glaucoma during periodic health examinations (grade C recommendation). The Task Force does state, however, that patients who have a family history of glaucoma, are black, have severe myopia, or have type 2 diabetes are at greatest risk of glaucoma. Thus, a cost-effective casefinding recommendation is to include periodic assessment by an optometrist or ophthalmologist with the frequency of assessment adjusted to patients’ risk of developing glaucoma.10 Various authors have suggested that ocular examinations should be conducted every 5 years from age 40 to 60 and every 3 years from age 60 on.11,12 Earlier and more frequent referrals can be made for patients with risk factors or as deemed appropriate by their primary eye-care providers. Recently, Huang et al13 demonstrated that only about half of family physicians screened for glaucoma; the main reason for not screening was lack of equipment or skill. Examination of the optic nerve head for characteristic structural glaucomatous changes is the mainstay of diagnosis. It has been demonstrated that for ophthalmologists, the sensitivity and specificity of this sign can exceed 90% for diagnosis of glaucoma.14,15 Measurement of IOP and visual field testing are also valuable.
Chronic open-angle glaucoma is an insidious, initially asymptomatic disease that accounts for 90% of all the different forms of glaucoma. It typically has adult onset and no symptoms until late in the disease when there is often bilateral, asymmetric loss of vision.4 For this reason it has been referred to as the “sneak thief of sight.” At least 50% of the nerve fibres could be lost by the time visual field deficits are evident.16 Family physicians’ referral of patients with risk factors to an optometrist or an ophthalmologist is crucial to diagnose glaucoma, and is currently the best way of ensuring early detection. Early detection and proper management with IOP-lowering therapy and follow up every 3 to 6 months for monitoring the IOP, the optic nerve, and periodically the visual fields, helps most of these patients to retain good visual function.17-19
Risk factors.
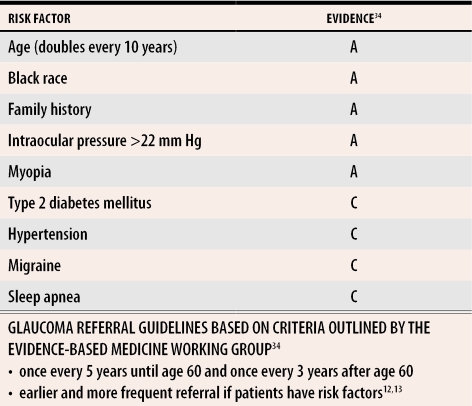
The five major risk factors for COAG are advanced age, black race, family history of glaucoma, myopia, and elevated IOP. Other risk factors with moderate-to-fair epidemiologic evidence include hypertension, type 2 diabetes, hypothyroidism, steroid use, migraine, and sleep apnea. The prevalence of glaucoma rises with age: approximately 1% in white patients younger than 40 and rising to 2% to 5% among those older than 75, with respective values of 1% and 11% among patients of African descent.2,20 In general, the percentage of those affected increases sharply among patients older than 50. African-American people have a higher incidence; the Baltimore Eye Survey revealed African-Americans to be four to five times more likely to have COAG than whites, with age-adjusted prevalence rates of 1.29% for whites and 4.74% for blacks.2 Optic nerve damage also tends to occur at least one decade earlier among blacks than among other racial groups. There is a strong genetic tendency for glaucoma, making family history an important part of the medical history. The relative risk of having COAG is increased approximately 3.7 times for people who have a sibling with COAG.21 Myopia or nearsightedness is also associated with optic nerve damage. It has been suggested that the larger eye and thinner wall in myopic patients could increase the susceptibility of the optic nerve to pressure damage.
Hypertension, type 2 diabetes, and hypothyroidism are concomitant diseases that have demonstrated an association with glaucoma. Corticosteroid use, by any route, is another risk factor for both elevations in pressure and cataract development, with yearly eye examinations recommended for patients using corticosteroids for periods longer than 4 weeks. An examination might be needed within several weeks of starting steroids if patients have one or more risk factors for elevated IOP with steroid use (ie, presence of COAG, family history of glaucoma, myopia, type 2 diabetes, or rheumatoid arthritis). As no direct treatment can address the underlying optic nerve or trabecular meshwork abnormalities associated with glaucoma, treatment instead focuses on lowering IOP.
Intraocular pressure.
Although elevated IOP is not considered by many glaucoma specialists to be part of the definition of glaucoma, it is the only major risk factor amenable to treatment. Mean IOP in normal eyes is 15 to 16 mm Hg (±2 standard deviation) with a range of 10 to 21 mm Hg and skewed in distribution to the higher pressures. Up to 10% of patients older than 40 have IOP above 21 mm Hg; those who have elevated pressures without optic nerve damage are termed ocular hypertensive or glaucoma suspects. Normal-tension glaucoma is defined as glaucomatous nerve damage and visual field loss with an IOP below 22 mm Hg. In fact, up to one in six patients with glaucoma have the normal-tension variety.22 Hence, because the relationship between IOP and visual damage is not necessarily constant, it is more useful to think of levels of IOP that patients’ optic nerves cannot withstand, regardless of their numerical value.
Management.
Medical therapy is usually the first line of treatment for COAG, with treatment directed at reaching and maintaining a preestablished “target pressure range” that is expected to halt optic nerve damage and visual field loss.17-19,23 Determining target pressure takes into account the severity of the optic nerve damage, patients’ baseline IOP, and (if known) the rapidity and progression of damage, as well as other risk factors for progression.
Medical treatments lower IOP by either reducing the ciliary body’s production of aqueous humour or enhancing aqueous outflow through the trabecular meshwork or uveoscleral pathways. Alternative methods for lowering IOP include applying laser energy to the trabecular meshwork to improve fluid outflow (laser trabeculoplasty) and surgical creation of a fistula from the anterior chamber to the subconjunctival space (trabeculectomy).
As topical treatments are more site-specific and have a lower incidence of systemic effects than oral medications, they are the mainstay of initial glaucoma treatment. Topical treatment is begun with a stepwise approach and takes into account patients’ ocular, systemic, and psychological profiles. A single topical drug is given up to its maximal dosage if well tolerated, before another agent is added. Other drugs are added based on their mechanisms of action and their complementary effects on lowering IOP. Thus, patients receiving maximal medical therapy could be using several different classes of glaucoma medications. Because of the brief contact with the ocular surface and the strong protective barrier of the eye, topical drops are concentrated. Drugs administered to the eye pass rapidly through the nasolacrimal duct into the nose, where they are absorbed into the highly vascular nasal mucosa and into systemic circulation. This systemic absorption is without the benefit of first-pass metabolism by the liver. Thus these drugs first circulate to the heart and then to the lungs. In fact, many ophthalmologists accept the dictum that patients taking topical beta-blockers receive systemic beta-blockade.
To decrease systemic absorption while increasing contact time with the ocular surface, patients should be instructed to occlude the nasolacrimal duct with either digital pressure or by simply closing their eyes for 5 minutes24 (Figure 3). If inserting multiple drops, it is advisable to wait about 5 minutes between application of each drop to avoid washing out the previous drop.
Figure 3. Nasolacrimal duct occlusion.
To decrease systemic absorption while increasing the contact with the ocular surface, patients should be instructed to occlude the nasolacrimal duct with either digital pressure or by simply closing their eyes for 5 minutes after applying topical medications.
Five major classes of drugs are used for long-term management of open-angle glaucoma: beta-adrenergic antagonists, hypotensive lipids, alpha2-adrenergic agonists, carbonic anhydrase inhibitors, and cholinergic agonists. In general, beta-adrenergic antagonists and carbonic anhydrase inhibitors decrease production of aqueous humour. Hypotensive lipids include prostaglandin and prostamide analogues, which primarily increase uveoscleral outflow, while cholinergics increase trabecular outflow. Finally, alpha2-adrenergic agents both decrease aqueous production and increase uveoscleral outflow.
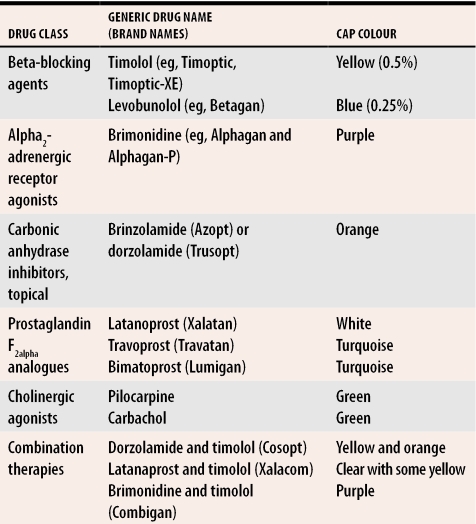
Many patients do not know the names of the eye drops they are using. Thus a helpful question is to ask them the cap colour of the bottle containing their eye drops (Table 2). Patients can also be asked to bring in a list of their medications, which pharmacies can often print off for them, or simply bring in their medications in paper or plastic bags.
Table 2. Cap colour of various glaucoma medications.
The systemic carbonic anhydrase inhibitors acetazolamide and methazolamide are pills, rather than eye drops.
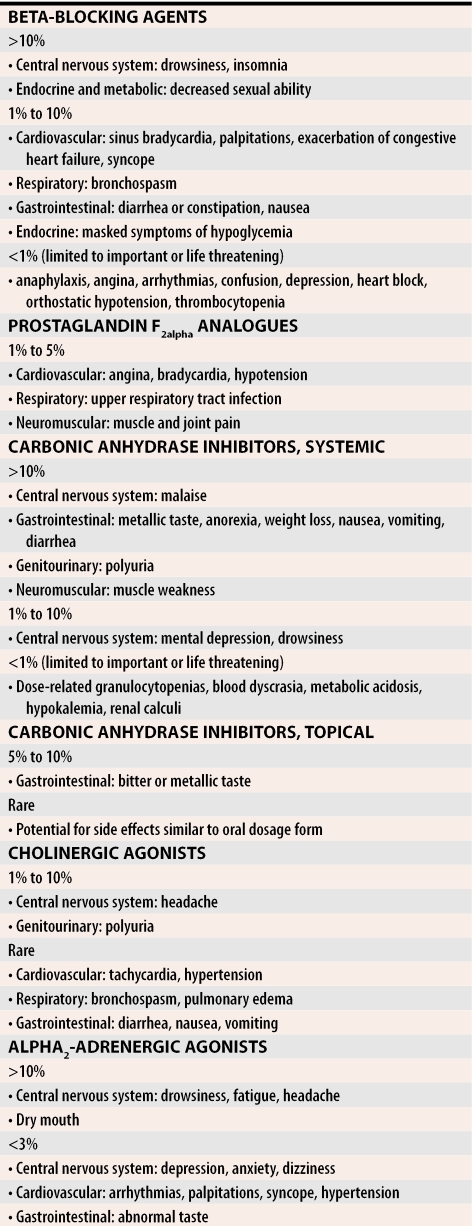
Although topical glaucoma medications are generally well tolerated, systemic side effects can be severe. The systemic side effects are notable, as they could cause a patient to present to a primary care physician and not to an eye-care specialist. Such common conditions as asthma, gastrointestinal or cardiovascular disturbances, depression, and sexual dysfunction can all present as side effects of drug treatment (Table 3).
Table 3.
Systemic side effects of the major classes of glaucoma medications
Perhaps the most common and dangerous side effects occur with beta-adrenergic antagonists, which often are the first drugs initiated in glaucoma treatment because of their pressure-lowering efficacy and relatively few ocular side effects.25 Beta2-blockade, however, can cause bronchial smooth muscles to contract, leading to bronchospasm and status asthmaticus, particularly among patients with asthma or chronic obstructive pulmonary disease.26-29 Cardiac side effects of beta-blockers include hypotension, decreased myocardial contractility, bradycardia, syncope, and worsening congestive heart failure.26,27,29,30
Carbonic anhydrase drugs are another class with serious side effects. Of note is the fact that these drugs are structurally related to sulfonamides; thus, renal failure, blood dyscrasias, and dermatologic reactions (including Stevens-Johnson syndrome) can occur.31-33 They should be avoided in patients with sulfa allergies.
Case revisited
Mrs F. is older than 50, is of African descent, is myopic, and suffers from diabetes and hypertension. All of these are risk factors for glaucoma, which should have prompted early referral. Inquiry would have revealed that she also has a family history of glaucoma. The history of asthma is important, as systemic side effects of topical medications used to lower eye pressure (such as beta-blockers) can exacerbate this problem and hence should be avoided. Family physicians might encounter patients with iatrogenic effects of treatment and thus should be aware of contraindications to, as well as side effects of, topical and systemic glaucoma therapy.
Conclusion
Chronic open-angle glaucoma is an insidious disease that is difficult to diagnose and affects people who are often unaware of its effects. Its damage is irreversible; appropriate referral and an understanding of treatment-related side effects are essential to prevent blindness and maintain quality of life. Effective treatments can arrest progression or slow the glaucomatous damage. Early diagnosis is the best way to maximize the number of years of sight for patients who have glaucoma. Current screening protocols suggest a thorough eye examination once every 5 years until age 60, and every 3 years after age 60, with earlier and more frequent examinations for those with risk factors (Table 434). Patients being treated for glaucoma are generally seen by their managing ophthalmologists every 3 to 12 months. Although topical medications are generally safe, systemic side effects can occur. Family physicians have a key role as gatekeepers in glaucoma surveillance and as safeguards against iatrogenic harm.
Table 4.
Glaucoma referral guidelines
Level A—strong research-based evidence, that is, multiple relevant, high-quality studies with homogeneous results (eg, two or more randomized controlled trials, or a systematic review with clearly positive results)
Level B—moderate evidence (eg, one randomized controlled trial or multiple adequate studies)
Level C—limited research-based evidence (eg, open, controlled, prospective studies)
Level D—no evidence (eg, retrospective studies or the consensus reached by an expert group in the absence of good-quality evidence)
Acknowledgments
Amy Bovell and Risa Desa provided helpful advice regarding this manuscript.
Biographies
Dr Adatia is an ophthalmology resident in the Department of Ophthalmology at the University of Toronto in Ontario.
Dr Damji is a glaucoma specialist in the Eye Institute at the University of Ottawa and is a researcher in glaucoma and genetics at the Ottawa Health Research Institute in Ontario.
Footnotes
Competing interests: None declared
References
- 1.Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980;24(Suppl):335–610. [PubMed] [Google Scholar]
- 2.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266:369–374. [PubMed] [Google Scholar]
- 3.Hitchings RA. Ocular surgery for the new millennium: glaucoma in the new millennium. Ophthalmol Clin North Am. 1999;12(4):519–531. [Google Scholar]
- 4.American Academy of Ophthalmology. Preferred practice pattern: primary angle-closure glaucoma. San Francisco, Calif: American Academy of Ophthalmology; 2000. [Google Scholar]
- 5.Lichter PR. Testimony before: Department of Labor, Health, and Human Services, and Related Agencies. 1st U. S. Congress House Committee on Appropriations for 1993. Washington, DC: Government Printing Office; 1992. [Google Scholar]
- 6.Johnson CA, Keltner JL. Incidence of visual field loss in 20,000 eyes and its relationship to driving performance. Arch Ophthalmol. 1983;101:371–375. doi: 10.1001/archopht.1983.01040010371002. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez P, Wilson MR, Johnson C, Gordon M, Cioffi GA, Ritch R, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol. 1997;115:777–784. doi: 10.1001/archopht.1997.01100150779014. [DOI] [PubMed] [Google Scholar]
- 8.Jocson VL, Sears ML. Experimental aqueous perfusion in enucleated human eyes. Results after obstruction of Schlemm’s canal. Arch Ophthalmol. 1971;86(1):65–71. doi: 10.1001/archopht.1971.01000010067013. [DOI] [PubMed] [Google Scholar]
- 9.Bill A, Phillips CI. Uveoscleral drainage of aqueous humour in human eyes. Exp Eye Res. 1971;12(3):275–281. doi: 10.1016/0014-4835(71)90149-7. [DOI] [PubMed] [Google Scholar]
- 10.Periodic health examination, 1995 update: 3. [cited 2005 May 25];Screening for visual problems among elderly patients. 152:1211–1222. Available from: http://www.ctfphc.org/ [PMC free article] [PubMed]
- 11.Boivin J, McGregor M, Archer C. Cost effectiveness of screening for primary open-angle glaucoma. J Med Screen. 1996;3:154–163. doi: 10.1177/096914139600300309. [DOI] [PubMed] [Google Scholar]
- 12.Tuck MW, Crick RP. The cost-effectiveness of various modes of screening for primary open angle glaucoma. Ophthalmic Epidemiol. 1997;4(1):3–17. doi: 10.3109/09286589709058056. [DOI] [PubMed] [Google Scholar]
- 13.Huang JT, Rhemtulla F, Huang PT. Glaucoma screening by primary care physicians in southern Alberta: patterns, methods and deficiencies. Can J Ophthalmol. 2003;38(4):279–284. doi: 10.1016/s0008-4182(03)80092-3. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JT. Methodologic differences and measurement of cup-disc ratio: an epidemiologic assessment. Arch Ophthalmol. 1976;94:1101–1105. doi: 10.1001/archopht.1976.03910040021004. [DOI] [PubMed] [Google Scholar]
- 15.Holmin C. Optic disc evaluation versus the visual field in chronic glaucoma. Acta Ophthalmol. 1982;60:275–283. doi: 10.1111/j.1755-3768.1982.tb08382.x. [DOI] [PubMed] [Google Scholar]
- 16.Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982;100:135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 17.Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 18.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 19.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 20.Target IOP Workshop participants. Damji KF, Behki R, Wang L. Canadian perspectives in glaucoma management: setting target intraocular pressure range. Can J Ophthalmol. 2003;38(3):189–197. doi: 10.1016/s0008-4182(03)80060-1. [DOI] [PubMed] [Google Scholar]
- 21.Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 22.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javit J, Singh K. Relationship between intraocular pressure and primary open-angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109(8):1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 23.Jampel HD. Target pressure in glaucoma therapy [review]. J Glaucoma. 1997;6(2):133–138. [PubMed] [Google Scholar]
- 24.Zimmerman TJ, Kooner KS, Kandarakis AS, Ziegler LP. Improving the therapeutic index of topically applied ocular drugs. Arch Ophthalmol. 1984;102:551–553. doi: 10.1001/archopht.1984.01040030429017. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen SJ, Abel SR. Comparison of the ocular beta-blockers [review]. Ann Pharmacother. 1996;30(1):43–54. doi: 10.1177/106002809603000109. [DOI] [PubMed] [Google Scholar]
- 26.McMahon CD, Shaffer RN, Hoskins HD, Jr, Hetherington J., Jr Adverse effects experienced by patients taking timolol. Am J Ophthalmol. 1979;88(4):736–738. doi: 10.1016/0002-9394(79)90674-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Buskirk EM. Adverse reactions from timolol administration. Ophthalmology. 1980;87(5):447–450. doi: 10.1016/s0161-6420(80)35215-9. [DOI] [PubMed] [Google Scholar]
- 28.Jones FL, Jr, Ekberg NL. Exacerbation of asthma by timolol. N Engl J Med. 1979;301(5):270. [PubMed] [Google Scholar]
- 29.Nelson WL, Fraunfelder FT, Sills JM, Arrowsmith JB, Kuritsky JN. Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, 1978-1985. Am J Ophthalmol. 1986;102(5):606–611. doi: 10.1016/0002-9394(86)90532-5. [DOI] [PubMed] [Google Scholar]
- 30.Leier CV, Baker ND, Weber PA. Cardiovascular effects of ophthalmic timolol. Ann Intern Med. 1986;104(2):197–199. doi: 10.7326/0003-4819-104-2-197. [DOI] [PubMed] [Google Scholar]
- 31.Howlett SA. Renal failure associated with acetazolamide therapy for glaucoma. South Med J. 1975;68(4):504–506. doi: 10.1097/00007611-197504000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Higenbottam T, Ogg CS, Saxton HM. Acute renal failure from the use of acetazolamide (Diamox). Postgrad Med J. 1978;54(628):127–128. doi: 10.1136/pgmj.54.628.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraunfelder FT, Meyer SM, Bagby GC, Jr, Dreis MW. Hematologic reactions to carbonic anhydrase inhibitors. Am J Ophthalmol. 1985;100(1):79–81. doi: 10.1016/s0002-9394(14)74987-6. [DOI] [PubMed] [Google Scholar]
- 34.Tuulonen A, Airaksinen PJ, Erola E, Forsman E, Friberg K, Kaila M. The Finnish evidence-based guideline for open-angle glaucoma. Acta Ophthalmol Scand. 2003;81:3–18. doi: 10.1034/j.1600-0420.2003.00021.x. [DOI] [PubMed] [Google Scholar]