Figure 3.
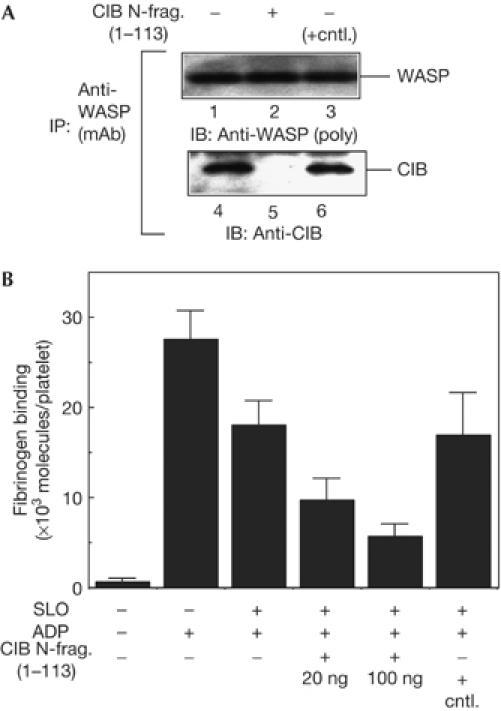
Blocking Wiskott–Aldrich syndrome protein binding to calcium- and integrin-binding protein reduces αIIbβ3 affinity for its ligand. (A) Streptolysin O (SLO)-permeabilized platelets were incubated with the calcium- and integrin-binding protein (CIB) amino-terminal fragment or a control protein and then stimulated with ADP without stirring in the presence of 100 μM of GRGDSP peptide. Wiskott–Aldrich syndrome protein (WASP) was immunoprecipitated from platelet lysates with anti-WASP monoclonal antibody followed by immunoblotting for WASP (lanes 1–3) and CIB (lanes 4–6). (B) SLO-permeabilized platelets (8 × 107 cells) were incubated with the CIB N-terminal fragment or 100 ng of FLAG–calcyclin as a control protein (cntl.) and then stimulated with ADP without stirring. Fibrinogen binding was measured as described in Methods. Data are the means±s.e. of three experiments.
