Figure 4.
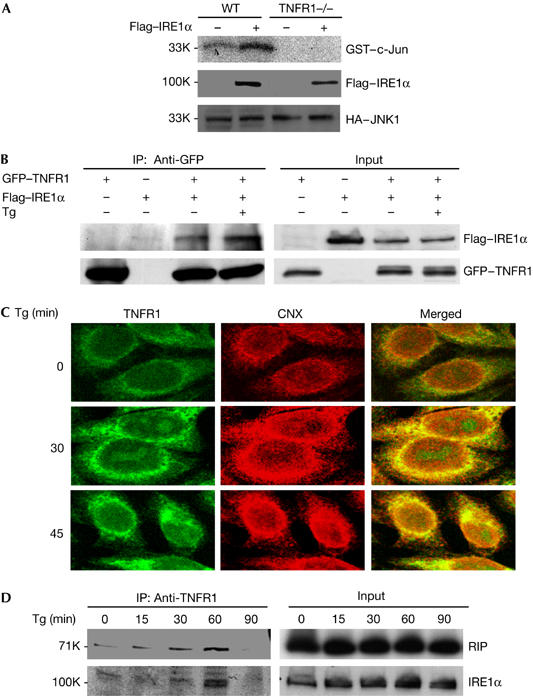
IRE1α interacts with TNFR1 and forms a complex with TNFR1 and RIP in response to ER stress. (A) WT and TNFR1−/− cells were co-transfected with plasmids HA–JNK1 and Flag–IRE1α overnight. Cell extracts were immunoprecipitated by anti-HA antibody, and then used for JNK kinase assay. (B) 293 cells were transiently transfected with plasmids GFP–TNFR1 and Flag–IRE1α, and then left without treatment or treated with Tg for 1 h. Cell extracts were used for immunoprecipitation with anti-GFP antibody. (C) The colocalization of TNFR1 with ER marker protein calnexin (CNX). WT cells were treated with 2 μM Tg for 30 or 45 min. The cells were immunostained with anti-TNFR1 antibody (R&D Systems) and anti-calnexin (CNX, BD). Confocal images indicate the colocalization of TNFR1 and CNX in ER in response to Tg treatment. (D) WT cells were treated with 2 μM Tg and collected for immunoprecipitation with anti-TNFR1 antibody. ER, endoplasmic reticulum; GFP, green fluorescent protein; HA, haemagglutinin; JNK, Jun amino-terminal kinase; RIP, tumour necrosis factor receptor-interacting protein; TNFR1, tumour necrosis factor receptor 1; Tg, thapsigargin; WT, wild type. Numbers at the left are relative molecular masses.
