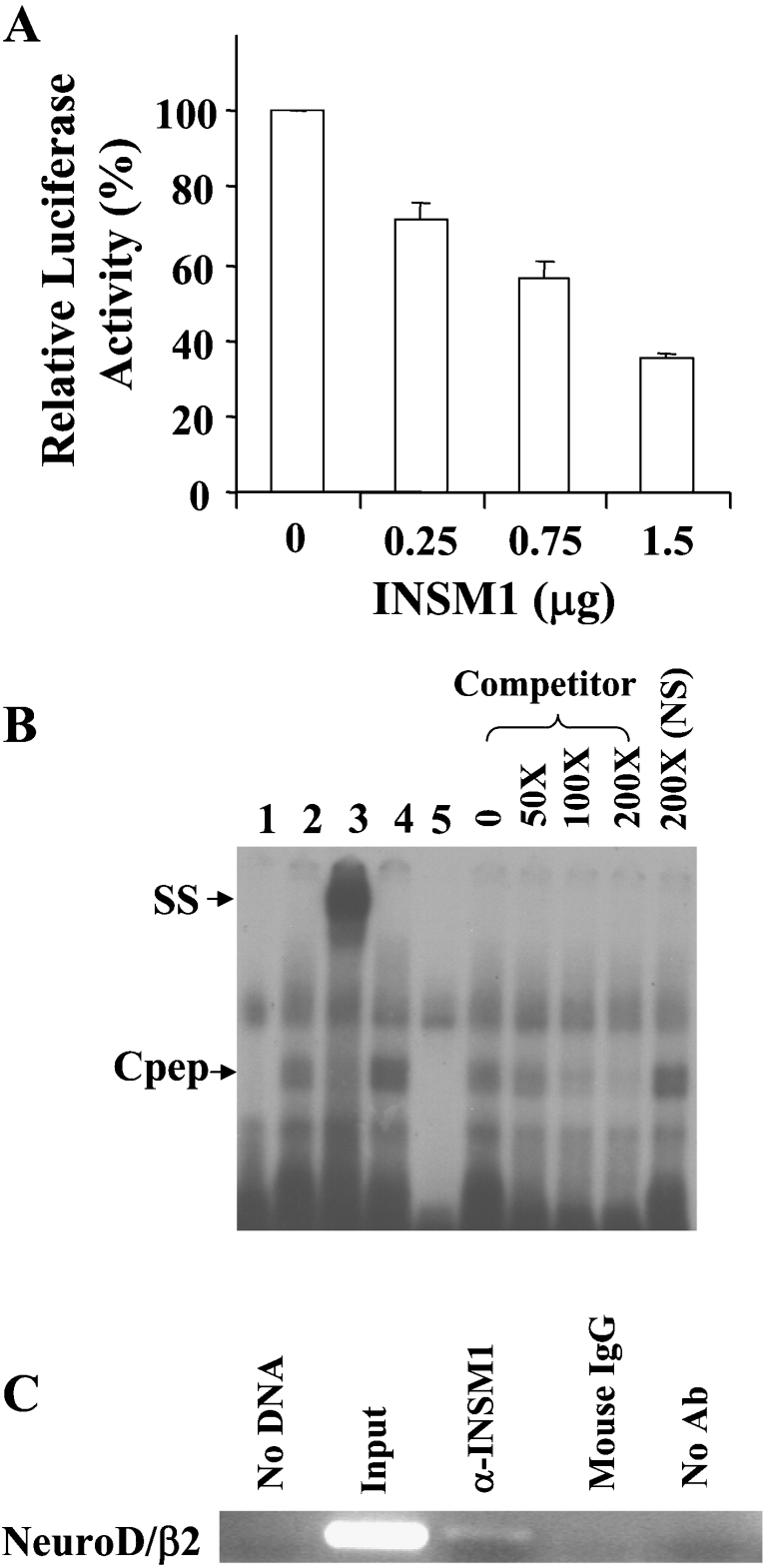
Figure 1. The neuroD/β2 promoter is a target gene suppressed by INSM1.
(A) Dose-dependent suppression of the neuroD/β2 promoter by INSM1 in HEK-293 cells. In HEK-293 cells, 1 μg of the mouse neuroD/β2 promoter (−419/+59 bp) linked to a luciferase reporter gene was co-transfected with increasing amounts (0.25–1.5 μg) of a CMV-INSM1 expression vector. Repressive effects on neuroD/β2 promoter activity using different amounts of CMV-INSM1 are shown as the percentage of suppression±S.E.M. The transfections were carried out and normalized in three sets of experiments. (B) EMSA. The double-stranded oligonucleotide spanning the −185 to −156 bp region of the mouse neuroD/β2 promoter, or a non-specific (NS) oligonucleotide was end-labelled as the probe. Probe alone (lane 1), with Cpep lysate (lane 2), Cpep lysate plus an anti-Cpep antibody (lane 3), Cpep lysate plus an anti-(mouse IgG) control antibody (lane 4), and the non-specific (NS) probe with the Cpep lysate (lane 5) are shown in the left of the panel. The right of the panel shows different concentrations (50–200×) of unlabelled competitor oligonucleotides. The presence of a specific band-shift (Cpep) and supershifted (SS) band indicated that the INSM1 C-terminal peptide (amino acids 257–510) is capable of binding to the INSM1 site in the neuroD/β2 promoter. (C) ChIP assay. Formaldehyde cross-linked chromatin from β-TC1 cells was incubated with a mouse antibody against INSM1 (lane 3). Immunoprecipitated genomic DNA was analysed by PCR using primers specific for the transcriptional regulatory sequences of the mouse neuroD/β2 (276 bp) promoter (see Materials and methods section). As a control, PCR reactions were carried out with no DNA (lane 1), input DNA (lane 2), DNA immunoprecipitated by normal IgG serum that was species-matched to the source of the test antibody (lane 4), and DNA that was immunoprecipitated in the absence of antiserum (lane 5). ChIP assays were repeated in two separate experiments. Ab, antibody.