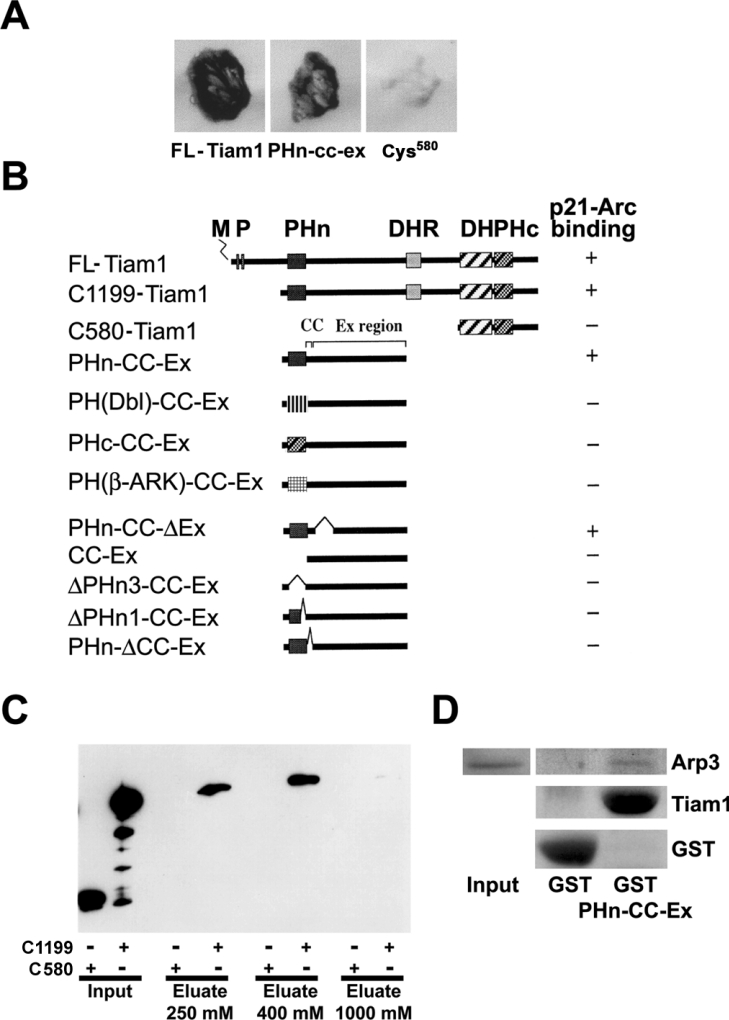
Figure 1. Tiam1 interacts with p21-Arc through the PHn domain and the adjacent CC region of Tiam1.
(A) Growth and β-galactosidase expression on 3AT/Trp−/Leu−/His− plates, in yeast transformed with the plasmid encoding p21-Arc, together with plasmids encoding the PHn-CC-Ex region of Tiam1, FLTiam1 or C580-Tiam1. (B) Comparison of Tiam1 domain organization and yeast two-hybrid results. +, An interaction with p21-Arc in the yeast two-hybrid assay, as measured by growth on 3AT/Trp−/Leu−/His− plates and β-galactosidase expression. −, No interaction in the yeast two-hybrid assay. M, myristoylation site; P, PEST (amino acid sequence enriched in proline, glutamic acid, serine and threonine) domain; DHR, discs large homology region; PHc, C-terminal PH domain [22]. PH domains from human Dbl and human β-Ark were used. (C) Crude cell lysates prepared from COS-7 cells (see input) expressing HA-tagged C1199- or C580-Tiam1 were passed over affinity chromatography columns containing bacterially expressed GST–p21-Arc. C1199-Tiam1, but not C580-Tiam1 is specifically retained on the column and is eluted at between 250 and 400 mM NaCl. Tiam1 proteins were detected using the anti-12CA5 antibody against the HA-tag. (D) The PHn-CC-Ex domain of Tiam1 is sufficient for Arp 3 binding. Purified GST and GST–PHn-CC-Ex were used for pull-down assays in COS-7 cell lysates, and Arp3-binding was determined by Western blot analysis.