Abstract
Cytokines are implicated in the anaemia of chronic disease by reducing erythropoiesis and increasing iron sequestration in the reticuloendotheial system. However, the effect of cytokines, in particular TNFα (tumour necrosis factor α), on small bowel iron uptake and iron-transporter expression remains unclear. In the present study, we subjected CD1 male mice to intraperitoneal injection with TNFα (10 ng/mouse) and then examined the expression and localization of DMT1 (divalent metal transporter 1), IREG1 (iron-regulated protein 1) and ferritin in duodenum. Liver and spleen samples were used to determine hepcidin mRNA expression. Changes in serum iron and iron loading of duodenum, spleen and liver were also determined. We found a significant (P<0.05) fall in serum iron 3 h post-TNFα exposure. This was coincident with increased iron deposition in the spleen. After 24 h of exposure, there was a significant decrease in duodenal iron transfer (P<0.05) coincident with increased enterocyte ferritin expression (P<0.05) and re-localization of IREG1 from the basolateral enterocyte membrane. Hepatic hepcidin mRNA levels remained unchanged, whereas splenic hepcidin mRNA expression was reduced at 24 h. In conclusion, we provide evidence that TNFα may contribute to anaemia of chronic disease by iron sequestration in the spleen and by reduced duodenal iron transfer, which seems to be due to increased enterocyte iron binding by ferritin and a loss of IREG1 function. These observations were independent of hepcidin mRNA levels.
Keywords: anaemia of chronic disease (ACD), ferritin, hypoferraemia, iron, iron-regulated protein 1 (IREG1), tumour necrosis factor α (TNFα)
Abbreviations: ACD, anaemia of chronic disease; Ct, threshold cycle; DMT1, divalent metal transporter 1; FAM, 6-carboxyfluorescein; IL, interleukin; IFN-γ, interferon γ; IREG1, iron-regulated protein 1; IRP, iron regulatory protein; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; Sp1, specificity protein 1; TAMRA, 6-carboxytetramethylrhodamine; TNFα, tumour necrosis factor α
INTRODUCTION
ACD (anaemia of chronic disease) or anaemia of chronic inflammation, as it is also commonly referred to, has long perplexed clinicians [1]. It is usually characterized by a normochromic normocytic anaemia with hypoferraemia and hyperferritinaemia in the face of adequate body iron stores [2]. The pathogenesis of ACD is linked to increased circulating levels of pro-inflammatory cytokines found in chronic inflammatory disorders [3]. Cytokines such as TNFα (tumour necrosis factor α), IL-1 (interleukin 1), IL-6 and IFN-γ (interferon γ) have a number of diverse actions on the erythropoietic and reticuloendothelial system, and have all been implicated in the pathogenesis of ACD [4].
The role of IL-6 in ACD is related to its ability to stimulate hepcidin expression, causing hypoferraemia by iron sequestration within the reticuloendothelial system and reducing intestinal iron absorption [5]. The hepatic antimicrobial peptide hepcidin has been measured previously in increased concentrations in the urine of patients with ACD secondary to sepsis [6,7]. Nemeth et al. [8] demonstrated that media from LPS (lipopolysaccharide)-stimulated macrophages induced hepcidin production from cultured human hepatocytes, an effect which was reversed by the addition of IL-6-neutralizing antibodies [8]. In both that study [8] and a further study by Kemna et al. [9], infusion of IL-6 or its induction by LPS infusion led to increased hepcidin levels and resultant hypoferraemia in healthy human subjects. Interestingly, neither study demonstrated an induction in hepatic hepcidin expression by TNFα.
There is considerable evidence in the literature supporting a role for TNFα in the anaemia of both chronic inflammation and malignancy [10,11]. In the context of rheumatoid arthritis, anti-TNFα therapy has been shown to improve haemoglobin concentrations in affected patients compared with controls [12]. In another study, bone marrow aspirates from rheumatoid arthritis patients had low levels of haematopoietic progenitor CD34 cells and increased rates of erythroid cell apoptosis [13]. These effects were reversed by in vitro treatment with anti-TNFα antibodies. In two mouse studies, TNFα administration caused hypoferraemia [14,15]. In the latter work, this was attributed to increased iron sequestration within macrophages [15]. A potential mechanism for macrophage iron sequestration was proposed by Ludewicz et al. [16], who demonstrated that TNFα stimulation up-regulated the cellular iron import protein DMT1 (divalent metal transporter 1) and reduced the iron exporter IREG1 (iron-regulated protein 1) in a human monocyte cell line.
The majority of work studying the effects of pro-inflammatory cytokines in ACD has focused on the erythropoietic and reticuloendothelial systems. The direct effects of cytokines on small bowel iron absorption have been less well studied. Over the last decade, the key proteins involved in inorganic iron trafficking across the small bowel epithelium have been identified [17]. Briefly, ferric iron is thought to be reduced to the ferrous form by duodenal cytochrome b at the brush border and is imported into the enterocyte by DMT1. Once in the cell, iron may be stored as ferritin or exported across the basolateral membrane by IREG1, which works in conjunction with the copper-linked oxidase hephaestin in generating ferric iron, which is transported in the plasma largely bound to transferrin.
In a previous study, we demonstrated that TNFα produced rapid changes in the expression and localization of DMT1, IREG1 and ferritin in both in vitro and ex vivo enterocyte model systems [18]. This ultimately led to iron sequestration within enterocytes and a reduction of iron export across a Caco-2 cell monolayer. In a similar study, Johnson et al. [19] demonstrated reduced DMT1 expression in Caco-2 cells following 72 h of TNFα exposure. Interestingly, a previous mouse study had shown that small intestinal intra-epithelial lymphocytes produced TNFα in response to dietary iron, an effect which appeared to be important in preventing hepatic iron overload [20]. This led us to propose that local intestinal responsiveness to TNFα could provide enterocyte targets, allowing reduced iron absorption in the face of systemic TNFα excess in chronic inflammation. The in vitro effects observed were hepcidin-independent. We therefore wished to extend this study to see whether the same effects could be observed in a whole organism.
In the present study, using wild-type mice, we investigated the effects of TNFα on small intestinal iron-transporter expression and localization, as well as iron absorption. In addition, we have studied the effects of TNFα on iron levels in serum, spleen and liver with concomitant hepcidin expression.
MATERIALS AND METHODS
Animal experiments
CD1 male mice (6-week-old; 28–32 g) (Charles Rivers Laboratories, Margate, Kent, U.K.), were subject to intraperitoneal injection with either 0.15 M NaCl (control) or TNFα (10 ng/mouse) in 0.15 M NaCl. At 3 or 24 h post-injection, mice were anaesthetized and killed by cervical dislocation. Duodenum, liver, spleen and serum were then collected. Serum iron analysis was performed by Dr L. Ford at the Department of Clinical Chemistry, Birmingham City Hospital, Birmingham, U.K. Small bowel, spleen and liver were divided equally into three portions for: (i) RNA extraction, (ii) protein extraction, and (iii) immunohistochemistry. All animal experiments were performed under the authority of a U.K. Home Office licence. Mice were fed CRM (combined rat and mouse) diet (Scientific Diet Supplies, Witham, Essex, U.K.).
Real-time PCR
RNA was extracted from small bowel, liver and spleen specimens using TRIzol® reagent with 1 μg of RNA subject to reverse transcription utilizing a reverse transcription kit (Promega). cDNA was then subject to real-time PCR as described previously [21]. Briefly, all reactions were allowed to take place using 18 S ribosomal RNA as an internal standard (PE Biosystems/Roche). Each experiment was performed in triplicate and the reaction mixture contained one of the following sets of probes and primers. (i) DMT1: probe, 5′-FAM (6-carboxyfluorescein)-CTGCATTCTGCCTTAGTCAAGTCTAGACAGCTAAAC-TA-MRA (6-carboxytetramethylrhodamine)-3′, forward primer, 5′-AGCTGTCATCATGCCACACAAC-3′, and reverse primer, 5′-GCTTCTCGAACTTCCTGCTTATTG-3′; (ii) IREG1: probe, 5′-FAM-AGGATTGACCAGTTAACCAACATCTTAGCCCCTAMRA-3′, forward primer, 5′-AGCAAATATGAATGCCACAATACG-3′, and reverse primer, 5′-CAAATGTCATAATCTGGCCAACAG-3′; (iii) ferritin: probe, 5′-FAM-CCAACGAGGTGGCCGAATCTTCCTT-TAMRA-3′, forward primer, 5′-GGAACATGCTGAGAAACTGATGAA-3′, and reverse primer, 5′-CATCACAGTCTGGTTTCTTGATATCC-3′; or (iv) hepcidin (HEPC1): probe 5′-FAM-CCTGAGCAGCACCACCTATCTCCATCA-3′, forward primer, 5′-CTGTCTCCTGCTTCTCCTCCTT-3′, and reverse primer, 5′-CTGCAGCTCTGTAGTCTGTCTCATC-3′, in the presence of 1× Mastermix (PE Biosystems), 50 nM 18 S 5′ and 3′ primers and 200 nM 18 S probe [5′-VIC™ (Applied Biosystems]/3′-TAMRA-labelled) and 0.25 μl of cDNA (equivalent to 12.5 ng of reverse-transcribed RNA) in a 25 μl reaction volume. Reactions without cDNA were included as negative controls. Gene expression was normalized to the 18 S probe and presented as ΔCt (threshold cycle) values. For each sample the mean of the three ΔCt values was calculated. Comparison of gene expression between control and treated samples was derived from subtraction of control ΔCt values from treatment ΔCt values to give a ΔΔCt value and relative gene expression was calculated as 2−ΔΔCt. Relative gene expression was normalized to 1.0 (100%) of controls. Each experiment was performed in triplicate.
Western blotting
Whole duodenum was cut longitudinally, and the epithelium was removed using a glass slide. The extract was then processed for Western blotting as described previously [21] with monoclonal antibodies against DMT1 (5 μg/ml, NRAMP24-A; ADI), IREG1 (5 μg/ml, MTP11-A; ADI), ferritin (1:500 ab16875; Abcam), TFR1 (1:500, A11130; Zymed Laboratories) or cytokeratin 19 (1:2000, IF-15; Oncogene Research Products), with the last used for normalization of epithelial protein loading. Immunoreactive bands were then subject to densitometry using NIH (National Institutes of Health) software Image 1.62. Before reprobing with the different antibodies, the membranes were incubated in stripping buffer containing 2% SDS and 100 mM 2-mercaptoethanol in 62.5 mM Tris/HCl, pH 6.8, for 30 min at 70 °C.
Immunocytochemistry
Sections (7 μm) of small bowel were processed for immunohistochemistry as described previously [21]. Briefly, sections were dewaxed and then incubated in 0.1% (v/v) Tween 20 containing 1 mM EDTA, pH 8.0, for 16 h at 65 °C. Sections were then incubated for 1 h with DMT1 (10 μg/ml), IREG1 (10 μg/ml) or ferritin (1:500). Immunoreactivity was detected using the avidin–biotinylated secondary-antibody method (Dako ABC kit) and visualized with diaminobenzidine reagent. Sections were counterstained with haematoxylin. Omission of primary antibody was employed as a negative control. Stained sections were scored independently by three observers (N.S., C.T. and T.H.I.) with regard to cellular localization. Paraffin sections were viewed under a Nikon Eclipse E600 microscope, and digital images were taken using a Nikon DXM1200F camera. Nikon ACT-1 version 2.62 software was used for image acquisition.
Perl's staining
Paraffin sections were dewaxed and incubated in 1% HCl containing 1% ferrous cyanate for 20 min. Sections were washed and counterstained with Neutral Red before visualization. Images were visualized as above.
Radiolabelled iron uptake assay
The length of the duodenum was tied off at both ends, and the duodenal segment was pre-washed with 0.15 M NaCl at 37 °C. The segment was then injected with 250 μM FeCl3 (Fe/nitrilotriacetate, 1:2, w/w) containing 5 μCi of radiolabelled iron (59FeCl3, specific activity 185 GBq/g; PerkinElmer) in physiological medium (125 mM NaCl, 3.5 mM KCl, 1 mM CaCl2, 10 mM MgSO4 and 10 mM D-glucose in 16 mM Hepes/NaOH buffer, pH 7.4). The chelator nitrilotriacetate was chosen as it has been used previously in iron-absorption studies and has been shown to form relatively stable complexes with iron [22–23]. Following a 10 min incubation, the duodenal segment was removed, and the remaining carcass was subject to γ-radiation counting using a high-resolution bulk sample counter. The count measured was termed mucosal transfer.
Statistics
All experimental errors are shown as 2 S.E.M. Statistical significance was calculated by use of the unpaired Student t test using SPSS version 10.0. Significance was accepted at P<0.05.
RESULTS
Determination of serum iron levels in control and TNFα-treated mice
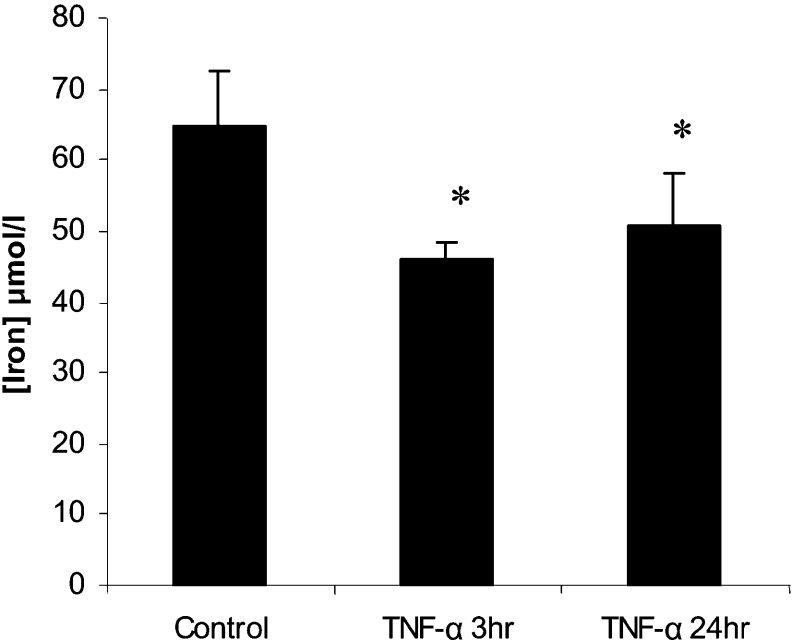
Analysis of serum iron levels in both 3 h and 24 h TNFα-treated mice demonstrated a significant decrease in serum iron compared with control mice (Figure 1).
Figure 1. TNFα causes a decrease in serum iron levels.
Male CD1 mice (6-week-old) were subject to intraperitoneal injection with either 0.15 M saline (control, n=6) or recombinant murine TNFα (10 ng/mouse, n=6). At 3 or 24 h post-intraperitoneal injection, blood was collected and serum were iron levels determined. *P<0.05. Results are means±2S.E.M.
TNFα-mediated repression of iron transfer across small bowel mucosa
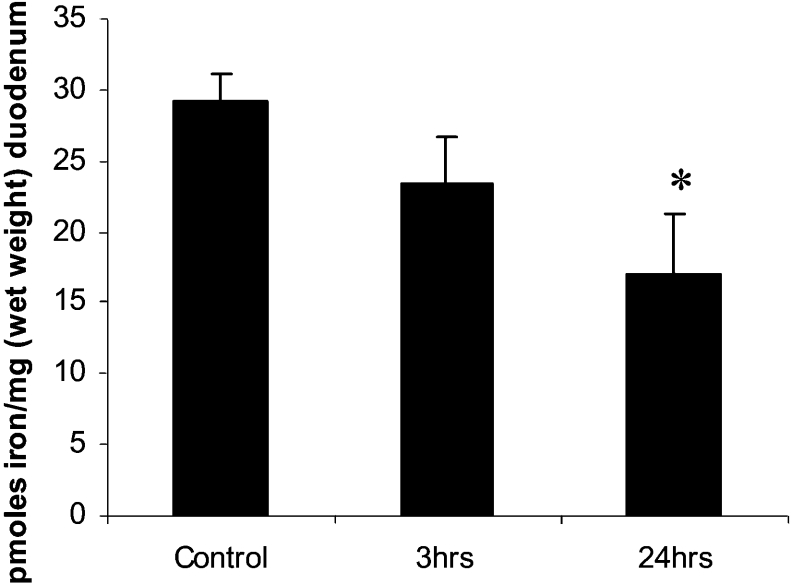
No significant change in mucosal iron transfer was observed after 3 h of stimulation with TNFα (Figure 2). However, a longer exposure to TNFα (24 h) significantly reduced mucosal transfer of iron compared with control mice (P<0.05).
Figure 2. TNFα-mediated repression in mucosal transfer.
Mucosal transfer (the amount of iron exported out of the duodenum) was determined in control, and 3 h and 24 h TNFα-treated mice. Results are means±2×S.E.M. for three independent experiments each performed in triplicate. *P<0.05.
Iron-transporter mRNA expression following TNFα exposure
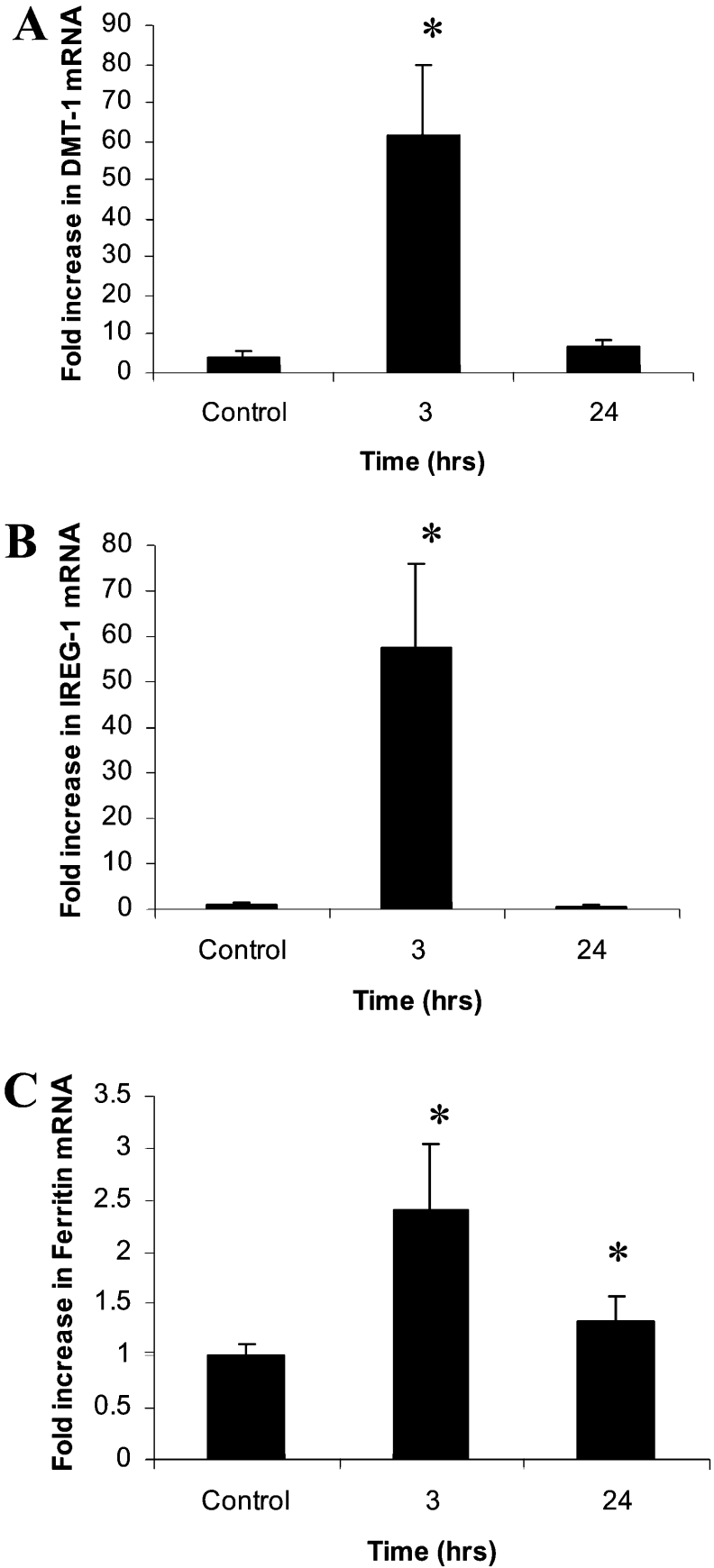
Consistent with our previous studies, real-time PCR analysis revealed a significant increase in DMT1, IREG1 and ferritin mRNA in 3 h TNFα-treated mice, which, with the exception of ferritin, had returned to baseline level by 24 h (Figure 3).
Figure 3. Modulation of DMT1, IREG1 and ferritin mRNA levels by TNFα.
CD1 male mice (6-week-old) were injected with either 0.15 M saline (control) or TNFα (10 ng/mouse). Mice were killed at either 3 h or 24 h post-injection, and duodenal mRNA levels of (A) DMT1, (B) IREG1 and (C) ferritin were determined. Results are means±2S.E.M. (n=6). *P<0.05.
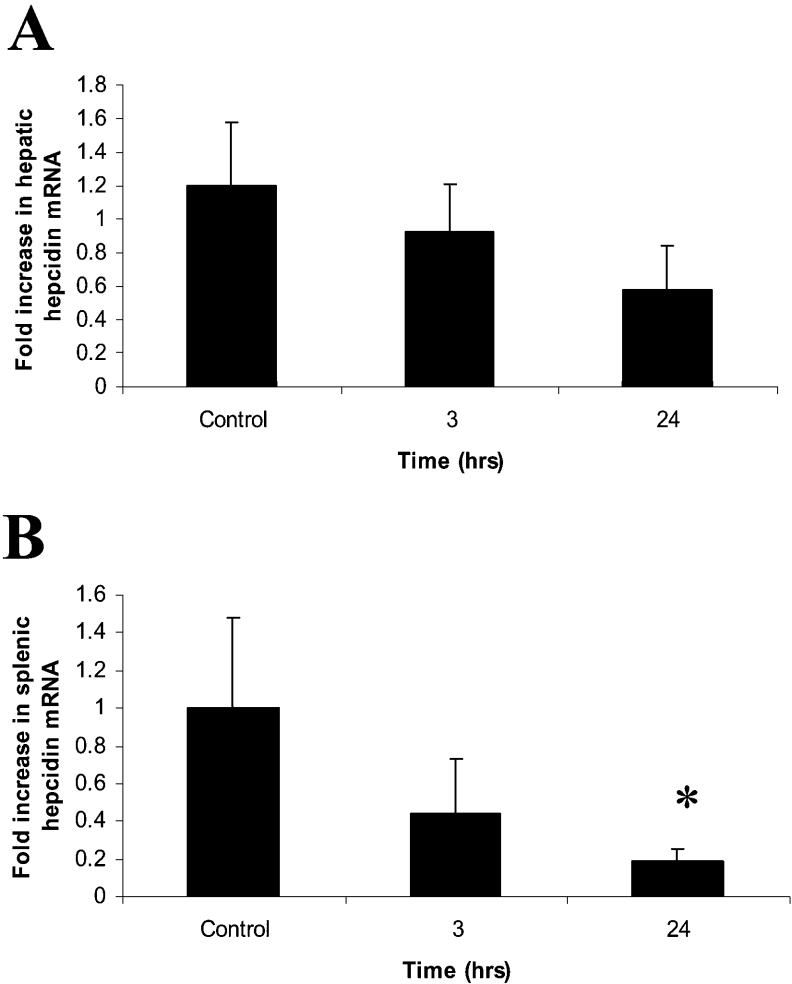
As hepcidin release has been shown to be a key mediator of the systemic response of iron metabolism to inflammation, we examined whether hepcidin was also modulated by TNFα. Livers and spleens from these mice were removed, RNA was extracted and real-time PCR was performed (Figure 4). In both 3 h and 24 h TNFα-treated mice, there was no significant modulation in hepatic hepcidin mRNA levels (Figure 4A). In contrast, splenic hepcidin levels appeared to be significantly repressed following 24 h of TNFα exposure (Figure 4B).
Figure 4. Modulation of hepcidin mRNA levels by TNFα.
CD1 male mice (6-week-old) were injected with either 0.15 M saline (control) or TNFα (10 ng/mouse). Mice were killed at either 3 h or 24 h post-injection, and hepatic (A) and splenic (B) mRNA levels of hepcidin were determined (n=6 for each time point). Results are means±2S.E.M. (n=6). *P<0.05.
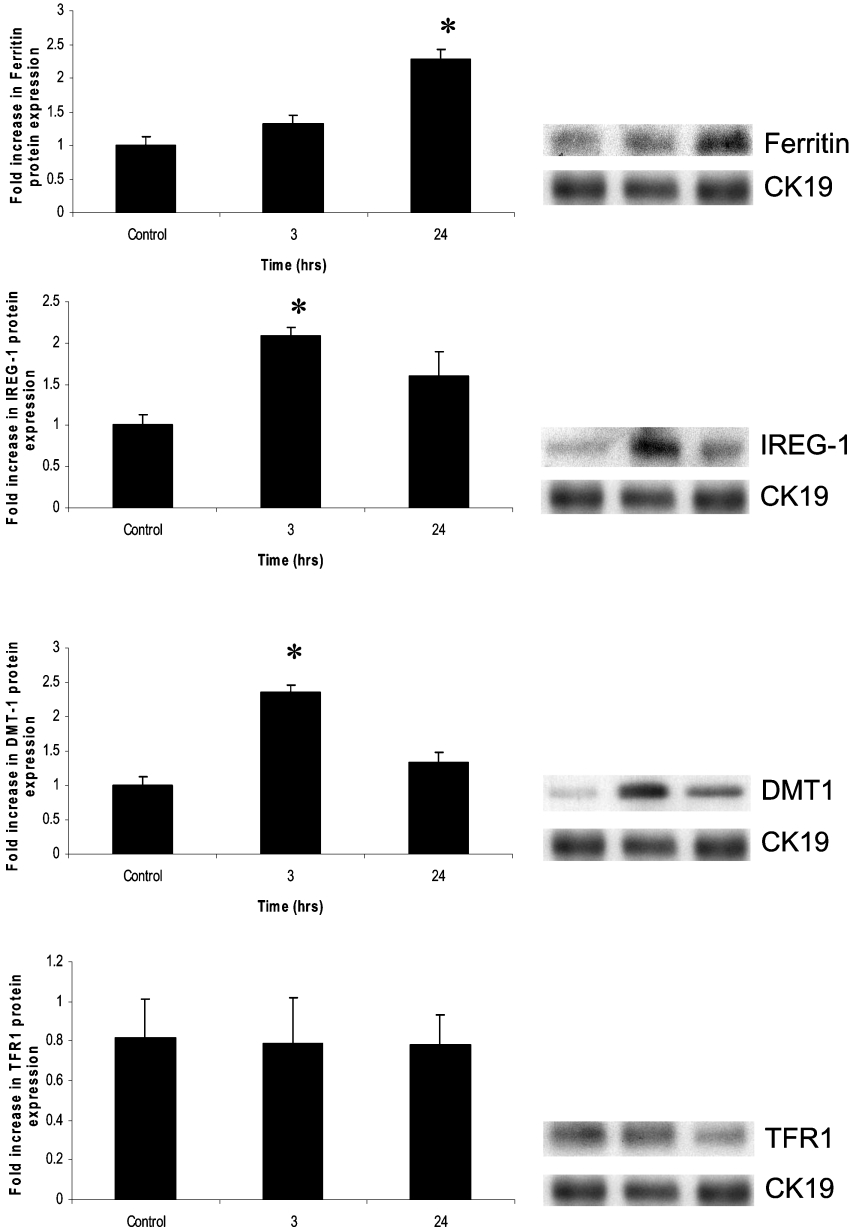
Iron-transporter protein expression following TNFα exposure
To determine whether these transcriptional changes were mirrored at the protein level, Western blotting was performed. In keeping with the mRNA data, at 3 h, there was a significant increase in DMT1 and IREG1 protein expression, although these had returned to baseline by 24 h (Figure 5). There was a comparative delay in ferritin expression, with protein levels only becoming significant at 24 h. There was no difference in the protein levels of TFR1 in mouse enterocytes at either time point, eliminating this as a potential route of iron ingress into these cells.
Figure 5. Protein expression of DMT1, IREG1, ferritin and TFR1 in response to TNFα stimulation.
At 3 h, there was significant increased expression of DMT1 and IREG1 protein. This was not maintained for 24 h. At 24 h, there was a significant increase in ferritin levels. There was no change seen in TFR1 expression. Cytokeratin 19 (CK19) was employed to ensure normalization of epithelial loading. Results are means±2S.E.M. (n=6). *P<0.05.
Immunolocalization of iron-transporter proteins
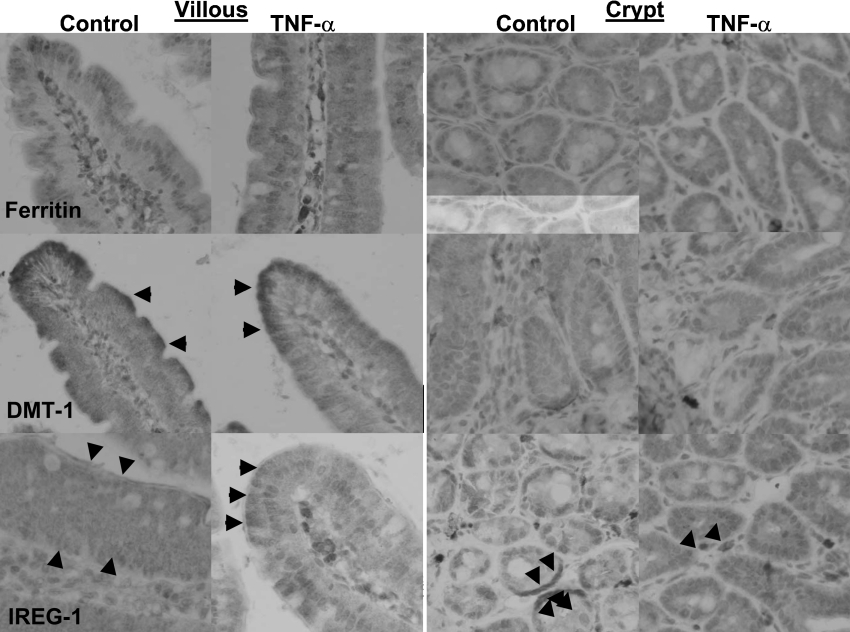
Furthermore, immunohistochemical studies showed alterations in the localization of iron-transporter proteins (Figure 6). Ferritin in control mice was localized predominantly in the apical portion of the villous enterocytes, while, in the treated animals, immunoreactivity throughout the enterocyte was apparent. No obvious difference was observed in the crypts, where ferritin immunoreactivity was weak and diffuse. The transporter DMT1 was localized as anticipated to the apical border of the villi in both control and TNFα-treated mice, and, in all mice, only very weak patchy cytoplasmic staining in the crypts was observed.
Figure 6. Immunolocalization of ferritin, DMT1 and IREG1 in control and TNFα-challenged mice.
Control and 24 h TNFα-treated mice (n=6 for each) were killed, and whole duodenum was processed into paraffin blocks, sectioned and subjected to immunohistochemistry with antibodies against DMT1 (NRAMP24A), IREG1 (MTP11-A) and ferritin (ab16875). Ferritin in control mice was localized predominantly to the apical pole of the villous enterocytes, while, in the TNFα-treated mice, immunoreactivity was observed throughout the cytoplasm of all villous enterocytes. Ferritin immunoreactivity was weak and diffuse in all crypts of both control and TNFα-treated mice. DMT1 was localized to the apical border of the villi in both control and TNFα-treated mice, as anticipated. However, in all mice, only very weak patchy cytoplasmic staining was observed in the crypts. IREG1 was observed in both apical and basal cytoplasmic compartments in control mice, while, in TNFα-treated mice, there was mostly apical cytoplasmic staining, with little evidence of basal immunoreactivity. In the crypts of control mice, IREG1 was preserved on the basolateral borders. This basolateral immunoreactivity was almost completely lost in the TNFα-treated mice, with only diffuse cytoplasmic immunoreactivity evident. Original magnification ×60. Arrowheads denote areas of positivity.
In control mice, there was evidence of both apical and basal cytoplasmic staining of IREG1 in the villous enterocytes, with the latter lost following TNFα treatment. In the crypts of control mice, IREG1 was predominantly preserved on the basolateral borders. This was almost completely lost, with only diffuse cytoplasmic immunoreactivity evident 24 h after TNFα treatment.
Perl's Prussian Blue staining of small bowel, spleen and liver
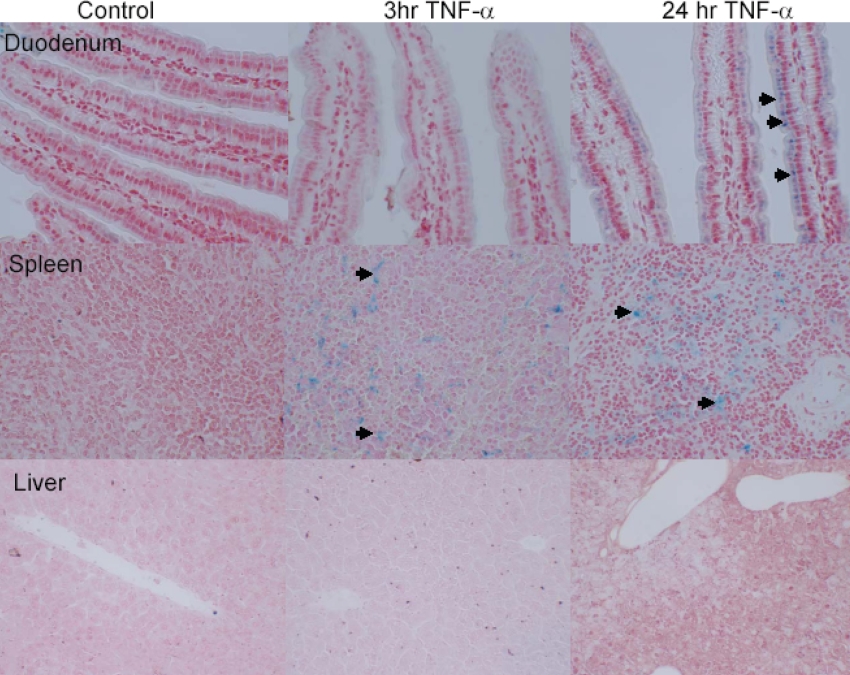
To examine whether these changes in expression and localization of ferritin, DMT1 and IREG1 led to an increase in enterocyte iron loading, Perl's staining was performed on duodenum from both control and TNFα-treated mice (Figure 7). In the villi of control and 3 h TNFα-treated mice there was no discernable staining; however, in the 24 h TNFα-treated mice, Prussian Blue staining was clearly observed in the apical poles of the villous enterocytes. In none of the mice was there any evidence of crypt Prussian Blue staining.
Figure 7. Enterocyte iron loading in TNFα-treated (24 h) mice.
Control mice showed no evidence of Prussian Blue staining in duodenum, spleen or liver. In both 3 h and 24 h TNFα-treated mice, an abundance of Prussian Blue staining was observed in both duodenal enterocytes and the spleen. However, no hepatic iron loading was observed in TNFα-treated mice. Original magnification ×40. Arrows denote areas of Perl's Prussian Blue staining.
To determine whether iron loading was confined to the small bowel, we chose to examine the two major tissue stores of iron, the spleen and liver (Figure 7). In the spleen, there was evidence of iron loading in both the 3 h and 24 h TNFα-treated mice, while there was no evidence of iron loading in the liver at either time point.
DISCUSSION
In a previous investigation using a Caco-2 cell model, we demonstrated that TNFα modulates iron transport, ultimately leading to inhibition of iron export from enterocytes [18]. These effects were local and hepcidin-independent. Our findings added to the evidence for a physiological role for TNFα in small bowel iron absorption and also suggested a potential mechanism for the inhibition of iron absorption in the context of systemic inflammation [20].
To clarify further the effects of TNFα on iron regulation, a mouse model was employed in the present study. In addition to assessing the effect of TNFα on iron-transporter expression and localization in enterocytes, we examined the simultaneous effects on liver and spleen.
In the present study, we have been able to demonstrate a rapid fall in serum iron 3 h after TNFα injection, which was coincident with an increase in splenic iron loading. It is likely that this effect was caused by increased retention of iron by monocytes in response to TNFα, as alluded to previously by others [15,16]. As expected, there was little or no parenchymal hepatic iron deposition in response to TNFα.
As in our earlier in vitro study [18], an early induction in both duodenal DMT1 and IREG1 was followed by a later fall to baseline levels along with a late up-regulation of ferritin. The induction of ferritin by TNFα is a recognized phenomenon and is likely to be an adaptive immune response to bacteraemia [24]. However, the relevance of the early induction of DMT1 and IREG1 is less obvious. Others have shown a similar increase in DMT1 expression in bronchial epithelial cells in response to TNFα, IFN-γ and LPS [25]. This increase in DMT1 expression was also paralleled by increased ferritin expression. The authors of that study speculated that the increase in DMT1 expression may be a local effect to ‘detoxify’ bronchial mucosa by reducing the access of potential micro-organisms to iron in the respiratory tract [25]. Whereas the early induction of iron-importing proteins at the intestinal brush border seen in the present study may seem counterintuitive, it remains possible that this early response is a similar attempt at local detoxification of small bowel luminal contents. Of note, despite the early up-regulation of both DMT1 and IREG1 mRNA and protein, no significant change in mucosal transfer of iron was seen at 3 h in TNFα-treated compared with control mice. This implies that, in enterocytes at least, the early induction in DMT1 and IREG1 protein is non-functional and is supported by the observation that there was no discernible enterocyte iron staining at 3 h.
The mechanisms that underlie the observed effects of TNFα on iron-transporter expression are unclear. Interestingly, the 5′-untranslated region of both ferritin and IREG1 genes reveal putative binding sites for the transcription factors NF-κB (nuclear factor κB) and Sp1 (specificity protein 1). Previous work has already implicated Sp1 in the activation of ferritin expression [26]. Two studies using fibroblasts and monocytic cell lines respectively have demonstrated a co-operative effect of Sp1 and NF-κB in the TNFα-dependent transcription of monocyte chemo-attractant protein 1 [27,28]. It is interesting to speculate whether a similar mechanism could be at play in our study. In contrast, the DMT1 promoter region lacks a NF-κB-binding domain, but does contain a putative IFN-γ-response element. TNFα has been shown previously to stimulate IFN-γ production in a number of cell types [29], and it is possible that the effects observed on DMT1 expression may be mediated by secondary IFN-γ release. It is also possible that the increase in DMT1 and IREG1 mRNA may be secondary to IRP (iron regulatory protein)-mediated posttranscriptional stabilization. However, IRP-mediated stabilization would normally lead to reciprocal changes in DMT1 and IREG1 expression.
In the present study, at 24 h, expression levels of DMT1 and IREG1 returned to baseline, while ferritin remained significantly induced. As in our previous in vitro observations, the ultimate effect of TNFα was to cause enterocyte iron loading and inhibition of iron absorption, which was independent of hepatic hepcidin expression. We propose that these effects are secondary to the induction of intracellular ferritin and the post-translational re-localization of IREG1 away from its basolateral compartment. Recently, work by Nemeth et al. [30] has demonstrated that hepcidin also exerts its major effect by binding to IREG1 and causing re-localization and loss of function of this iron exporter. It is therefore intriguing that, although TNFα and hepcidin appear to act independently in our model, their ultimate targets and effects are likely to be similar.
Liu et al. [31] have shown that LPS causes a post-translational down-regulation of IREG1 in murine splenic macrophages, which is IL-6- and hepatic hepcidin-dependent. Interestingly, their study also revealed that the transcriptional control of IREG1 in splenic macrophages was IL-6- and hepcidin-independent and that hepcidin was also synthesized in the spleen.
The relevance of splenic hepcidin production is unknown. In the present study, we have demonstrated that TNFα causes a significant repression in splenic hepcidin mRNA expression at 24 h. It therefore appears that TNFα is able to cause hypoferraemia independent of both hepatic and splenic hepcidin expression.
In summary, we provide evidence that TNFα can potentially cause ACD by the inhibition of small bowel iron absorption in addition to its documented effects on erythropoeisis and the reticuloendolethial system. This appears to be independent of hepcidin, but involves effects on enterocyte ferritin expression and IREG1 localization.
ACD is an important clinical problem with significant prognostic implications. To date, there have been few direct clinical strategies to treat this problem. It has been proposed that hepcidin antagonists may be developed in the future therapy for this condition [4]. In addition to this, TNFα antagonists, which are in widespread clinical use in conditions such as Crohn's disease and rheumatoid arthritis, present a possible future therapy for ACD.
Acknowledgments
This work was supported by grants from the BBSRC (Biotechnology and Biological Sciences Research Council) (BBS/B/10781) and Sandwell and West Birmingham NHS (National Health Service) Trust R&D fund. We thank Dr L. Ford of Birmingham City Hospital for serum iron analysis.
References
- 1.Locke A., Main E. R., Roschbach D. O. The copper and non-hemoglobinous iron contents of the blood serum in disease. J. Clin. Invest. 1932;11:527–542. doi: 10.1172/JCI100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16:87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 3.Means R. T. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells. 1995;13:32–37. doi: 10.1002/stem.5530130105. [DOI] [PubMed] [Google Scholar]
- 4.Weiss G., Goodnough L. T. Anaemia of chronic disease. N. Eng. J. Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 5.Andrews N. C. Anemia of inflammation: the cytokine–hecidin link. J. Clin. Invest. 2004;113:1251–1252. doi: 10.1172/JCI21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E., Valore E. V., Territo M., Schiller G., Lichtenstein A., Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 7.Kemna E., Tjalsma H., Laarakkers C., Nemeth E., Willems H., Swinkels D. Novel urine hepcidin assay by mass spectrometry. Blood. 2005;106:3268–3270. doi: 10.1182/blood-2005-05-1873. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B. K., Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemna E., Pickkers P., Nemeth E., van der Hoeven H., Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 10.Spivak J. L. The anaemia of cancer: death by a thousand cuts. Nat. Rev. Cancer. 2005;5:543–555. doi: 10.1038/nrc1648. [DOI] [PubMed] [Google Scholar]
- 11.Bertero M. T., Caligaris-Cappio F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica. 1997;82:375–381. [PubMed] [Google Scholar]
- 12.Davis D., Charles P. J., Potter A., Feldmann M., Maini R. N., Elliott M. J. Anaemia of chronic disease in rheumatoid arthritis: in vivo effects of tumour necrosis factor α blockade. Br. J. Rheumatol. 1997;36:950–956. doi: 10.1093/rheumatology/36.9.950. [DOI] [PubMed] [Google Scholar]
- 13.Papadaki H. A., Kritikos H. D., Valatas V., Boumpas D. T., Eliopoulos G. D. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-α antibody treatment. Blood. 2002;100:474–482. doi: 10.1182/blood-2002-01-0136. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T., Araki E., Nitta K., Tateno M. Recombinant human tumor necrosis factor depresses serum iron in mice. J. Biol. Response Modif. 1987;6:484–488. [PubMed] [Google Scholar]
- 15.Alvarez-Hernandez X., Liceaga J., McKay I. C., Brock J. H. Induction of hypoferremia and modulation of macrophage iron metabolism by tumor necrosis factor. Lab. Invest. 1989;61:319–322. [PubMed] [Google Scholar]
- 16.Ludwiczek S., Aigner E., Theurl I., Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 17.Sharma N., Butterworth J., Cooper B. T., Tselepis C., Iqbal T. H. The emerging role of the liver in iron metabolism. Am. J. Gastroenterol. 2005;100:201–206. doi: 10.1111/j.1572-0241.2005.40152.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma N., Laftah A. H., Brookes M. J., Cooper B. T., Iqbal T. H., Tselepis C. A role for tumour necrosis factor α in human small bowel iron transport. Biochem. J. 2005;390:437–446. doi: 10.1042/BJ20050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson D., Bayele H., Johnston K., Tennant J., Srai S. K., Sharp P. Tumour necrosis factor α regulates iron transport and transporter expression in human intestinal epithelial cells. FEBS. Lett. 2004;573:195–201. doi: 10.1016/j.febslet.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 20.Ten Elshof A. E., Brittenham G. M., Chorney K. A., Page M. J., Gerhard G., Chorney M. J. Gamma delta intraepithelial lymphocytes drive tumor necrosis factor-α responsiveness to intestinal iron challenge: relevance to hemochromatosis. Immunol. Rev. 1999;167:223–232. doi: 10.1111/j.1600-065x.1999.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 21.Tselepis C., Morris C. D., Wakelin D., Hardy R., Perry I., Luong Q. T., Harper E., Harrison R., Attwood S. E., Jankowski J. Upregulation of the oncogene c-myc in Barrett's adenocarcinoma: induction of c-myc by acidified bile acid in vitro. Gut. 2003;52:174–180. doi: 10.1136/gut.52.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laftah A. H., Ramesh B., Simpson R. J., Solanky N., Bahram S., Schumann K., Debnam E. S., Srai S. K. Effect of hepcidin on intestinal iron absorption in mice. Blood. 2004;103:3940–3944. doi: 10.1182/blood-2003-03-0953. [DOI] [PubMed] [Google Scholar]
- 23.Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J. Biol. Chem. 1967;242:2810–2815. [PubMed] [Google Scholar]
- 24.Torti F. M., Torti S. V. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Garrick M. D., Yang F., Dailey L. A., Piantadosi C. A., Ghio A. J. TNF, IFN-γ, and endotoxin increase expression of DMT1 in bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L24–L33. doi: 10.1152/ajplung.00428.2003. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji Y., Torti S. V., Torti F. M. Activation of the ferritin H enhancer, FER-1, by the cooperative action of members of the AP1 and Sp1 transcription factor families. J. Biol. Chem. 1998;273:2984–2992. doi: 10.1074/jbc.273.5.2984. [DOI] [PubMed] [Google Scholar]
- 27.Boekhoudt G. H., Guo Z., Beresford G. W., Boss J. M. Communication between NF-κB and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J. Immunol. 2003;170:4139–4147. doi: 10.4049/jimmunol.170.8.4139. [DOI] [PubMed] [Google Scholar]
- 28.Ping D., Boekhoudt G., Zhang F., Morris A., Philipsen S., Warren S. T., Boss J. M. Sp1 binding is critical for promoter assembly and activation of the MCP-1 gene by tumor necrosis factor. J. Biol. Chem. 2000;275:1708–1714. doi: 10.1074/jbc.275.3.1708. [DOI] [PubMed] [Google Scholar]
- 29.Nagano Y. Endotoxin induction of interferon. Tex. Rep. Biol. Med. 1977;35:105–110. [PubMed] [Google Scholar]
- 30.Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 31.Liu X. B., Nguyen N. B., Marquess K. D., Yang F., Haile D. J. Regulation of hepcidin and ferroportin expression by lipopolysaccharide in splenic macrophages. Blood Cells Mol. Dis. 2005;35:47–56. doi: 10.1016/j.bcmd.2005.04.006. [DOI] [PubMed] [Google Scholar]