Figure 3.
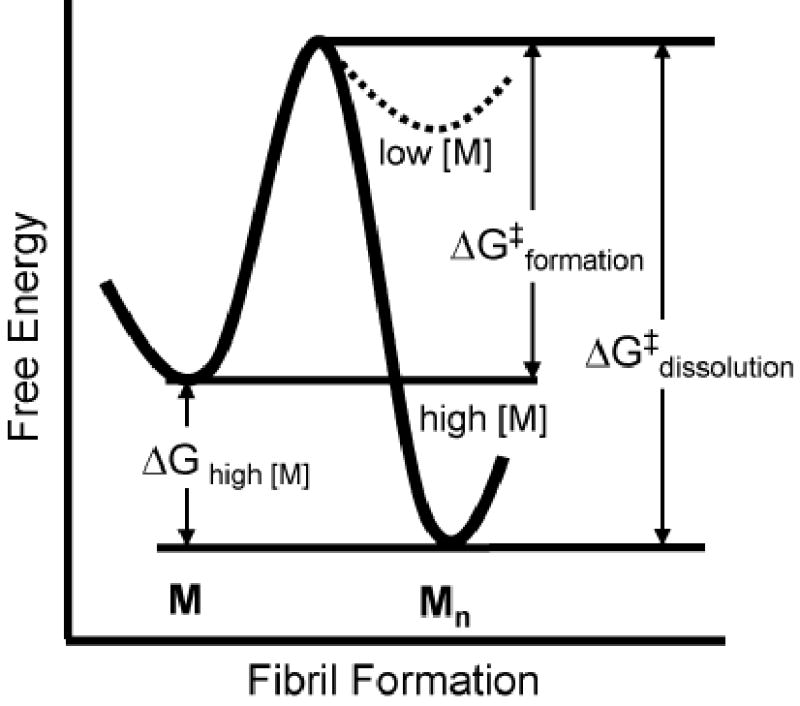
A conjectural plot of the free energy, G, for conversion of monomeric GNNQQNY, M, to the aggregated state, Mn. The standard free energy change ΔG0 for the conversion is small, so that the change in ΔG is controlled mainly by the concentration of monomer. At low concentrations, the monomeric state is favoured over the aggregated state, and the aggregated state is favoured at high concentrations. There is a significant kinetic barrier to formation of the aggregated state, ΔG‡ formation. At high concentrations of protein, the barrier to redissolve fibers, ΔG‡ dissolution, is very large.
