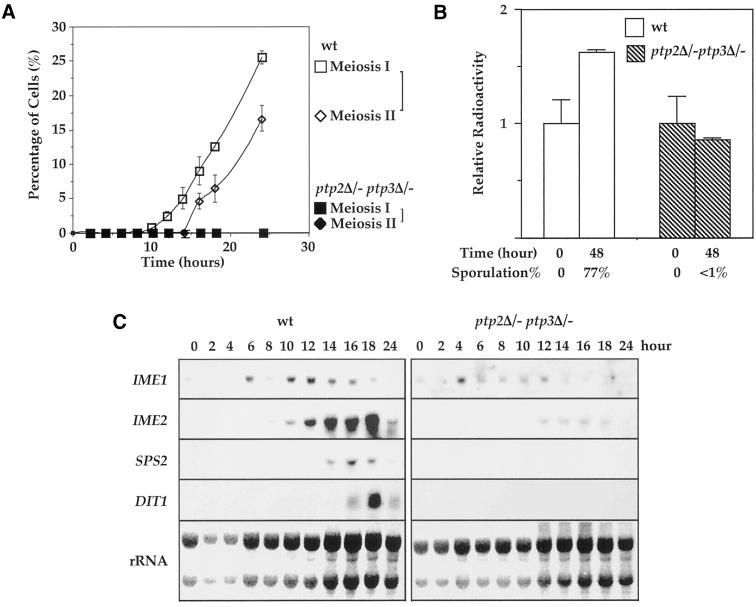
Figure 2.
ptp2Δ/− ptp3Δ/- deletion cells were blocked before pre-meiotic DNA synthesis and induction of sporulation specific genes was decreased in double deletion cells. (A) ptp2Δ/− ptp3Δ/− double deletion cells are arrested before meiosis I in sporulation medium. Wild-type (Y264) and ptp2Δ/− ptp3Δ/− (Y164) cells were withdrawn from sporulation medium at the indicated time points. Cells were stained with DAPI and examined by fluorescence microscopy to determine the percentage of cells that had completed meiosis I or meiosis II. Tetranucleate cells (also include trinucleate cells) were counted as cells that completed meiosis II, whereas cells with more than one nucleus were counted as cells that completed meiosis I. Open square, meiosis I in wild type; open diamond, meiosis II in wild type; closed square, meiosis I in ptp2Δ/− ptp3Δ/−; closed diamond, meiosis II in ptp2Δ/− ptp3Δ/−, respectively. (B). Premeiotic DNA synthesis is absent in ptp2Δ/− ptp3Δ/− double deletion cells. Wild-type diploid yeast cells (Y264, open bars) or ptp2Δ/− ptp3Δ/− (Y164, closed bars) were grown in presporulation medium with [14C]uracil to label the endogenous pool of nucleotides. Cells were shifted into sporulation medium for 48 h in the absence of [14C]uracil. DNA was isolated, and total radioactivity incorporated in DNA was determined by scintillation counting. In parallel, the sporulation efficiency of the same cell samples was determined by microscopic examination. Average and SD from duplicated assays are shown. (C) Expression of sporulation-specific genes is decreased in ptp2Δ/− ptp3Δ/− double deletion cells. RNA was prepared from either wild-type (Y264, left panels) or ptp2Δ/− ptp3Δ/− double deletion cells (Y164, right panels) taken at various times after transferred to sporulation medium. Northern hybridization was performed on total RNA with probes derived from IME1, IME2, SPS2, and DIT1 genes as indicated. The amount of total RNA loaded in each lane was visualized with methylene blue staining for rRNA (bottom panels).