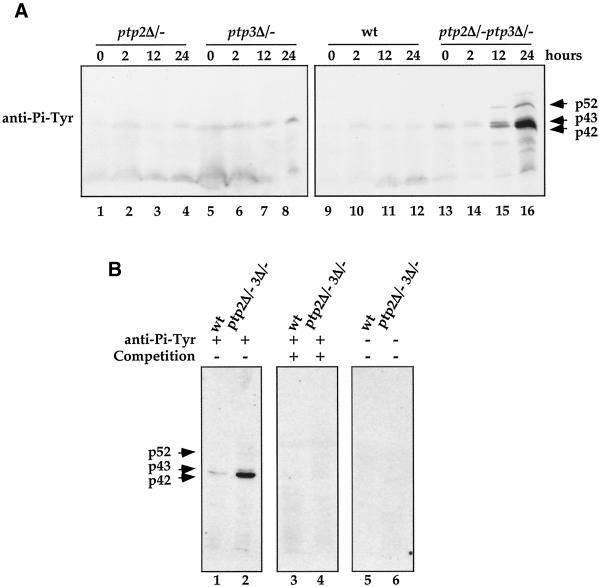
Figure 3.
Elevated protein tyrosine phosphorylation in ptp2Δ/− ptp3Δ/− double deletion cells during sporulation. (A) Tyrosine phosphorylation of 52-, 43-, and 42-kDa proteins is up-regulated in ptp2Δ/− ptp3Δ/− double deletion cells during sporulation. Total cellular lysates were prepared from wild type (Y264, lanes 9–12), ptp2Δ/− single deletion (Y162, lanes 1–4), ptp3Δ/− single deletion (Y163, lanes 5–8), and ptp2Δ/− ptp3Δ/− double deletion (Y164, lanes 13–16) grown in sporulation medium for various times. Equal amount of lysates were resolved on SDS-PAGE and subjected to immunoblotting with anti-Pi-Tyr antibody. The prominent tyrosine-phosphorylated proteins were denoted p42, p43, and p52 according to their apparent molecular masses. (B) Competition of anti-Pi-Tyr Western blots. Cell lysates prepared from wild-type (Y264) or ptp2Δ/− ptp3Δ/− double deletion cells (Y164) incubated in sporulation medium for 24 h were subjected to anti-Pi-Tyr Western blotting (lanes 1 and 2). The immunoreactive signals were specifically competed if the anti-Pi-Tyr antibody was preincubated with 1 mM phosphotyrosine (lanes 3 and 4). As a control, no signal was detected on Western blotting without primary anti-Pi-Tyr antibody (lanes 5 and 6).