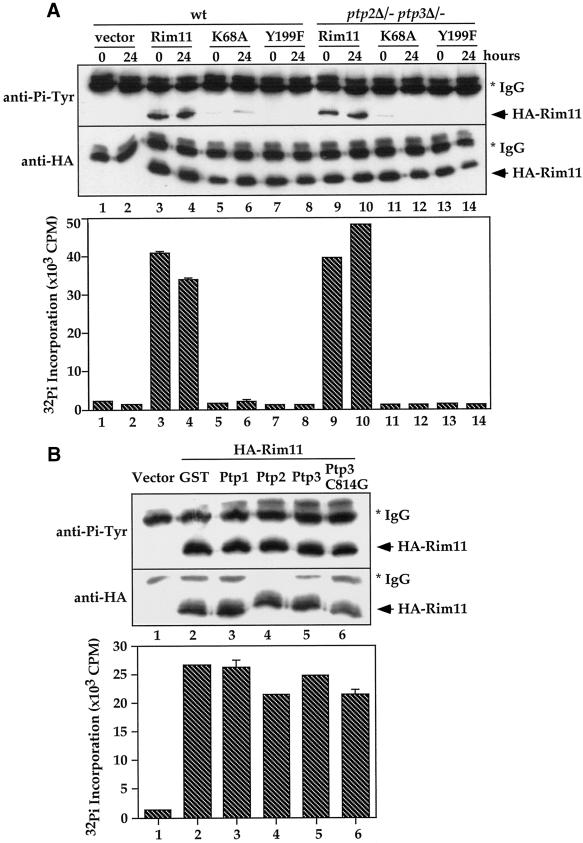
Figure 5.
Rim11 Tyr phosphorylation and activity are not affected by ptp2Δptp3Δ deletion or Ptp2 and Ptp3 overexpression. (A) Rim11 Tyr phosphorylation and kinase activity are not affected by ptp2Δ ptp3Δ deletion. Wild-type (Y264, lanes 1–8) or ptp2Δ/− ptp3Δ/− double deletion cells (Y164, lanes 9–14) containing vector (pRS313, lanes 1–2), HA-tagged Rim11 wild type (pXZ352, lanes 3, 4, 9, and 10), K68A (pXZ353, lanes 5, 6, 11, and 12), or Y199F (pXZ354, lanes 7, 8, 13, and 14) were harvested at 0 and 24 h after being shifted to sporulation medium. HA-tagged proteins were immunoprecipitated and subjected to anti-Pi-Tyr (top) or anti-HA immunoblotting (middle) to determine Tyr phosphorylation or protein levels, respectively. In parallel, immunoprecipitated proteins were subjected to in vitro kinase assay using phospho-GS peptide as a substrate to determine the kinase activity (bottom). The radioactivity incorporated into the peptide was measured by scintillation counting. Average results from duplicated kinase assays are shown. (B) Overexpression of Ptp2 and Ptp3 has no effect on Rim11 Tyr phosphorylation and kinase activity. Wild-type cells (Y264) harboring HA-Rim11 (pXZ352) were transformed with vector (lane 1), pXZ134 (lane 2), pXZ110 (lane 3), pXZ113 (lane 4), pXZ123 (lane 5) or pXZ136 (lane 6) to overexpress GST or the indicated PTPases. Cellular lysates were prepared from transformants growing in SC-His,Ura, and HA-Rim11 was immunoprecipitated. Tyr phosphorylation (top), protein levels (middle), and kinase activities (bottom) were determined.